Hydrogen is an important industrial commodity. It is used as a chemical feedstock for the production of petrochemicals, steel, glass and fertilisers; as an energy vector for clean transport systems; as a domestic combined heat and power; and as a potential storage medium for grid levelling. For all these applications a zero-emission method for the production is necessary to meet carbon emission targets, and the need is urgent.
Many companies, (e.g., Hitachi) and local authorities have aimed to be carbon neutral by 2030, an ambitious target to be met in less than a decade and requiring a large step change in production. One important method that meets this requirement is the electrolysis of water, using renewable or, at least, zero carbon electricity. However, to meet these ambitious targets, a rapid acceleration of research and development of hydrogen production technologies is needed.
CIC energiGUNE is already involved in research into the production of hydrogen by thermochemical cycles and is looking to increases its effort in the whole hydrogen value chain.
Hydrogen’s “colours”
Hydrogen can be produced by using energy to split water producing the two constituent elements of hydrogen and oxygen. This energy can be heat, light, electrical energy, or chemical energy, or any combination of these.
Conventional hydrogen production comes in three “colours” depending upon the amount of carbon released in the production process. The most common current method is by the steam reforming of methane, producing what is called “grey” hydrogen with the emission of carbon dioxide into the atmosphere. If the carbon dioxide produced by steam reforming is sequestered by Carbon Capture and Storage (CCS), then “blue” hydrogen is produced.
The question we ask above is how to produce “green” hydrogen using a source of renewable energy and no carbon emissions.
Electrolysis technologies
Electrolysis has its roots in the 19th century experiments into electrochemistry. In fact, electrolysers have been developed over the last 100 years and fall into three main categories: alkaline water electrolysis (AWE), Polymer Electrolyte Membrane Water Electrolysis (PEMWE), and high temperature Solid Oxide Electrolysis Cell (SOEC).
The most mature of these technologies, is AWE with large commercial units available, and up to 100MW units have been demonstrated. However, they suffer from problems of efficiency and poor start/stop dynamics, making them difficult to couple to renewable energy sources.
More recently, development of PEMWE has made some considerable progress and a UK company, ITM power, has become the world’s largest PEMWE manufacturer. ITM have developed 2 MW PEM modules and plan to install a 20 MW unit at Scottish Power Renewables’ (part of the Iberdrola group of companies) Whitelee Wind Farm near Glasgow to fuel hydrogen powered fuel cell busses as part of Glasgow’s goal to become a net zero city by 2030.
One of the drawbacks of PEM technology is the use of iridum and platinum as catalysts in the electrodes. Both are rare elements, with iridium being a particular concern, as annual production is only a few tons, which could limit large scale adoption at the terawatt scale needed to meet ambitious carbon reduction targets.
The least developed is the SOEC, this operates at temperatures typically between 600°C and 850°C and more correctly should be termed steam electrolysis. This is inherently more efficient than water electrolysis and can be especially advantageous where waste process heat is available. SOECs can also provide a route towards the production of liquid fuels for transport, taking advantage of current transport infrastructure. The co-electrolysis of carbon dioxide and water is used to produce syngas, a mixture of hydrogen and carbon monoxide (a feedstock for liquid fuel production); this provides a closed loop for carbon usage and has many advantages provided that low cost durable SOECs can be developed.
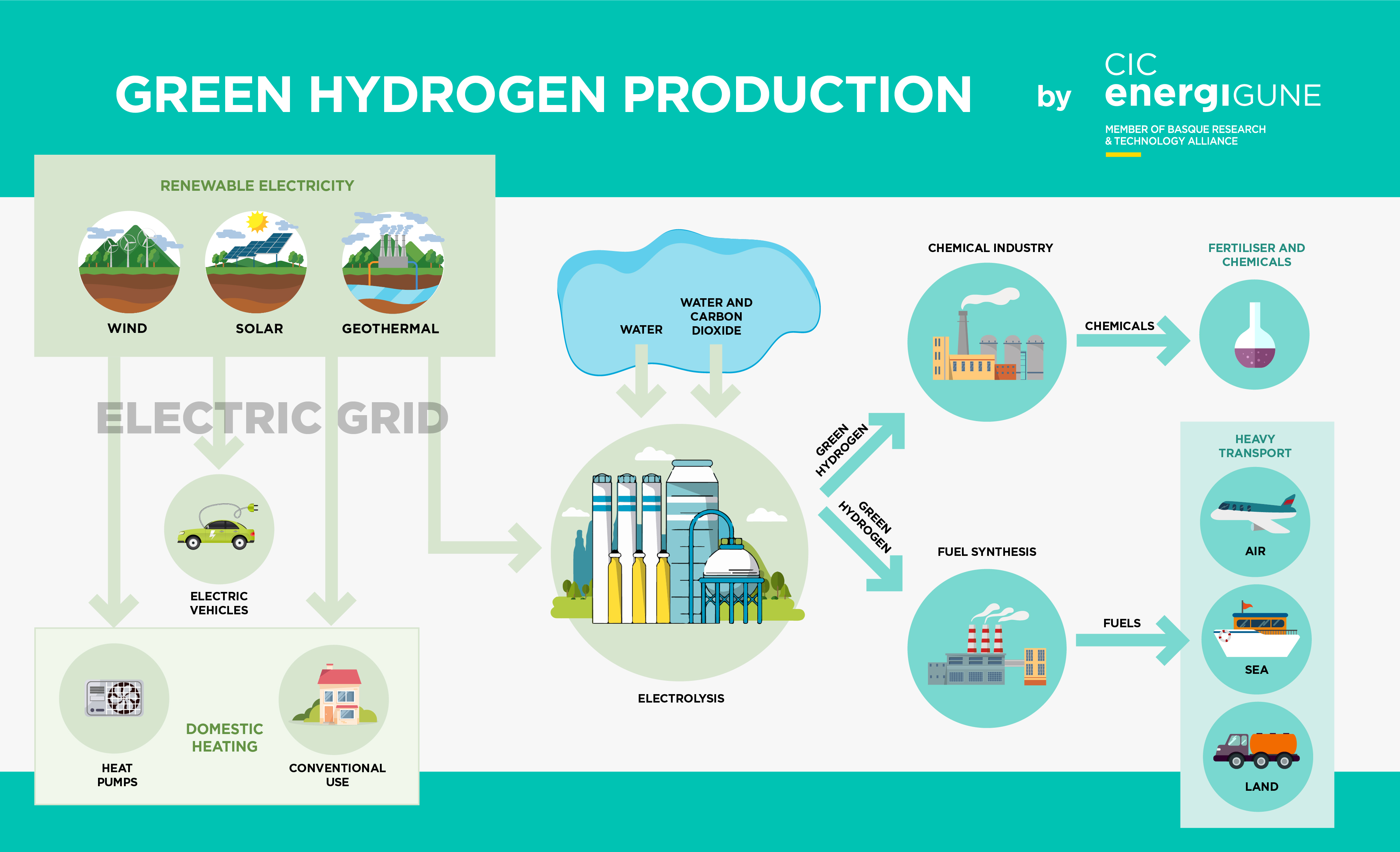
We are well on the way to achieving this goal. The diagram below shows the integration of electrolysis into a zero-emission future energy system.