This is the objective of the HIGREEW project coordinated by CIC energiGUNE, which has allowed the integration of an "Aqueous organic redox flow batteries" prototype in a renewable energy plant. Molecular engineering and computational tools are fundamental elements in this quest to probe the vast chemical space comprising these batteries.
In previous articles we have already given a glimpse of how these batteries work and the advantages that their particular design implies. These advantages include the decoupling of power and energy, the widespread use of aqueous solutions as safe non-flammable electrolytes, and the excellent stability and durability of their components, which also contributes to a lower environmental impact.
The sustainability of these batteries was also addressed in another of our articles and is a key aspect in the massive implementation of energy storage systems, as envisaged in the European Commission´s agenda.
Aqueous Organic flow batteries
Beyond the durability of the materials, the impact of the extraction of the materials or the manufacture of the cell components must be considered. With the electrolyte being the most abundant component of the battery and the one with the greatest environmental impact, the focus is on the chemistry of these batteries.
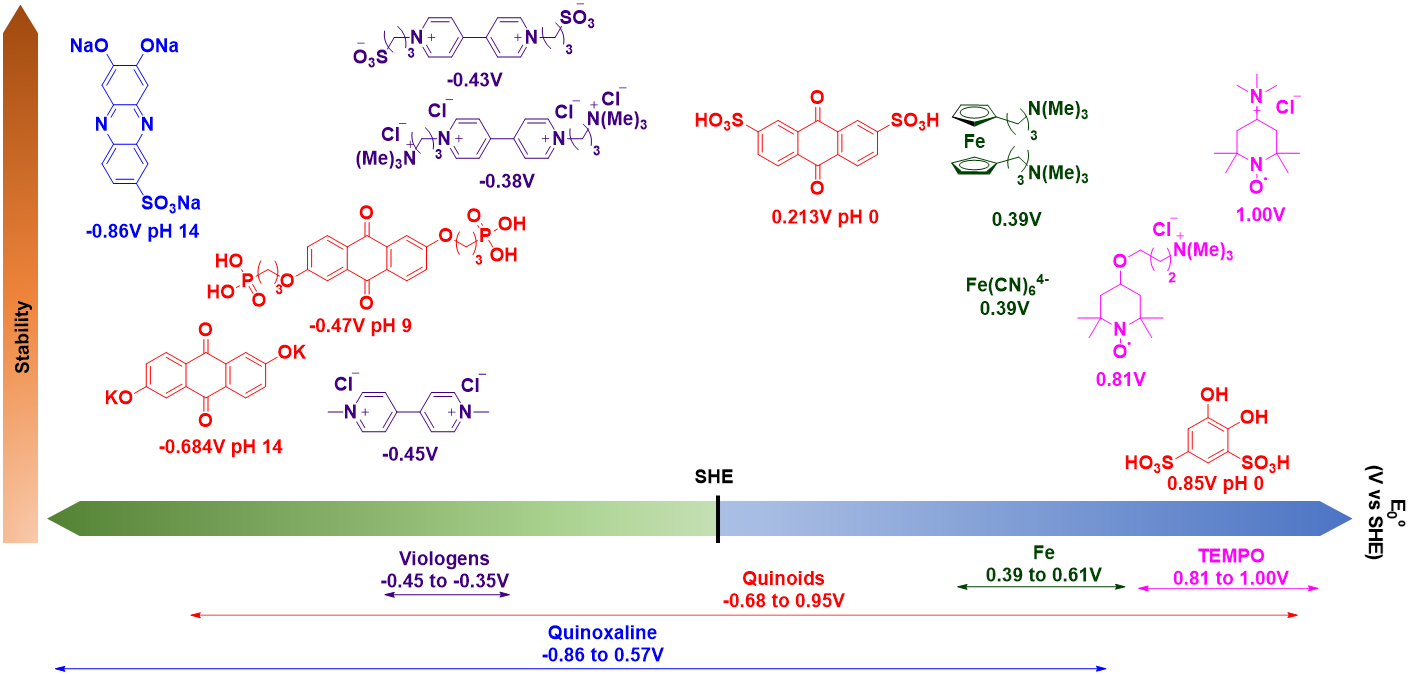
In this sense, it could be said that batteries based on organic materials have the irrefutable argument of the high availability of raw materials; not in vain, these materials consist mainly of Carbon, Hydrogen, Nitrogen and Oxygen.
The design of materials has been based on the knowledge of organic chemistry and nature as a source of inspiration. Thus, compounds of great relevance in the chemistry of living beings, such as alloxazines or quinones, which participate in metabolic and respiration mechanisms, respectively, have been modified for use in batteries.
Molecular engineering has made it possible to introduce structural changes to preserve the activity of these compounds while defining their properties. Stability, redox potential and water solubility are the key aspects that define the efficiency and energy density of flow batteries. Thus, salt formation is a widespread strategy to improve solubility.