Why the Shift from Lithium-ion?
Traditionally, lithium-ion batteries have been the workhorse of energy storage, meeting society´s promising energy demands. Unfortunately, they cannot achieve the required transformative scale of improvement required because of the: (i) limited geographical supply of Lithium (thus increase in cost), (ii) scarcity of cobalt and (iii) limited energy storage capacity.
Their widespread adoption faces significant hurdles, dampening their potential for large-scale implementation. Herein enters the attraction of Sodium-air/O2 batteries.
Unveiling the Potential of Na-air/O2 batteries
Na-air/O2 batteries offer a paradigm shift in energy storage dynamics. Na-air/O2 batteries directly address the issues pointed above due to: (i) the large abundance of sodium (Na) and its low cost (~30 times cheaper than their Lithium counterparts), (ii) the lack of requirement for cobalt, and (iii) their high theoretical energy density as a result of using a lightweight air cathode.
Na-air/O2 batteries present ca. 5–10 times higher theoretical capacities than that of Li-ion and Na-ion batteries (1,605/1,108 vs 100–265 and ~150 Wh kg-1, respectively) since the active material is taken from the surrounding environment (O2).
Operating Principles and mechanisms of Na-air/O2 batteries
A Na-air/O2 battery comprises three key elements: a sodium metal anode, a porous air cathode, and an electrolyte acting as a separator between them. The functionality of superoxide-based Na-O2 batteries primarily follows equations (I) and (II):
Na+ + O2 + e− ↔ NaO2 (Eº = 2.27 V) (I)
2Na+ + O2 + 2e− ↔ Na2O2 (Eº = 2.33 V) (II)
The initiation of the oxygen reduction reaction (ORR) typically involves a one-electron reduction process, yielding superoxide anions (O2-) that react with sodium metal cations (Na+) from the anode oxidation reaction to form the discharge product (NaO2). During discharge, the sodium metal anode oxidizes, releasing Na+ ions, while the cathode facilitates the ORR using available oxygen at the triple phase boundaries (air electrode/O2/electrolyte).
Two main mechanisms, the solution-mediated and surface-mediated mechanism, are proposed for NaO2 discharge product formation. Additionally, the selection of electrolyte is widely recognized as critical, as the shape, size, and formation mechanism of NaO2 discharge product strongly rely on the physicochemical properties of the electrolyte.
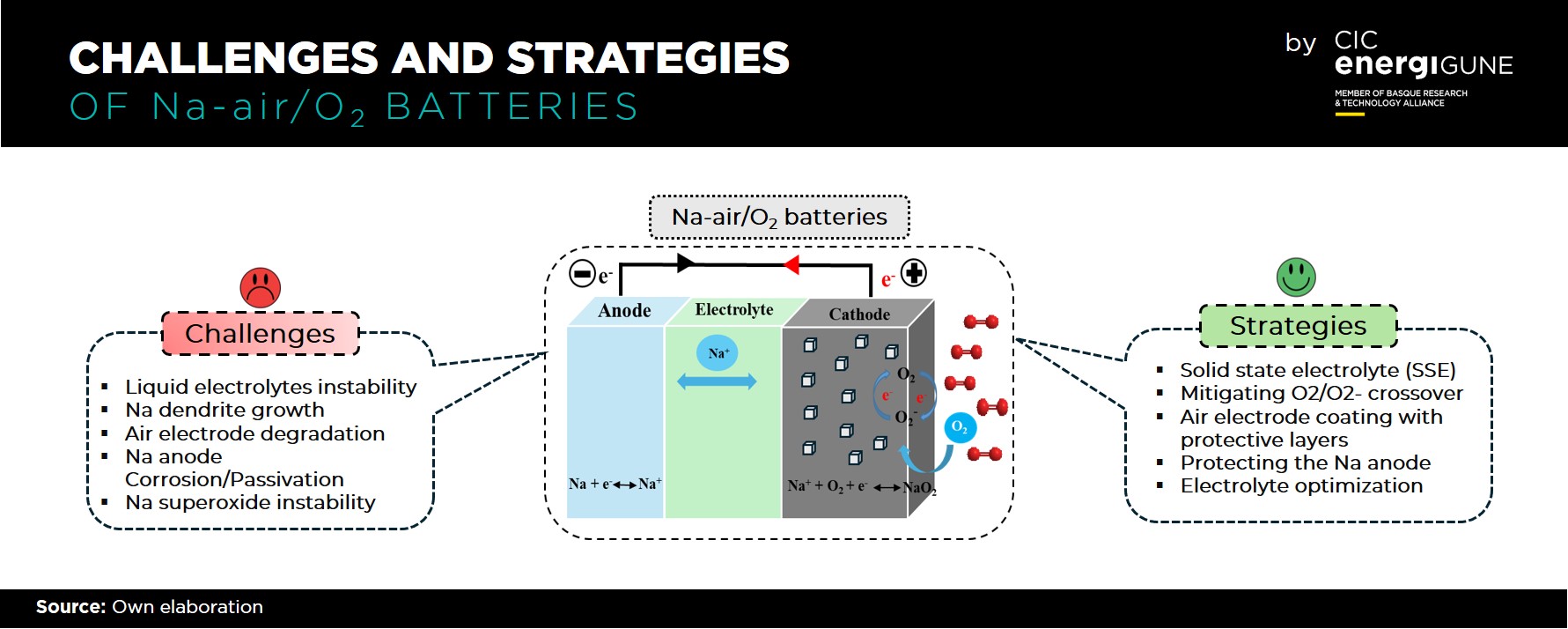
Addressing Challenges Head-On
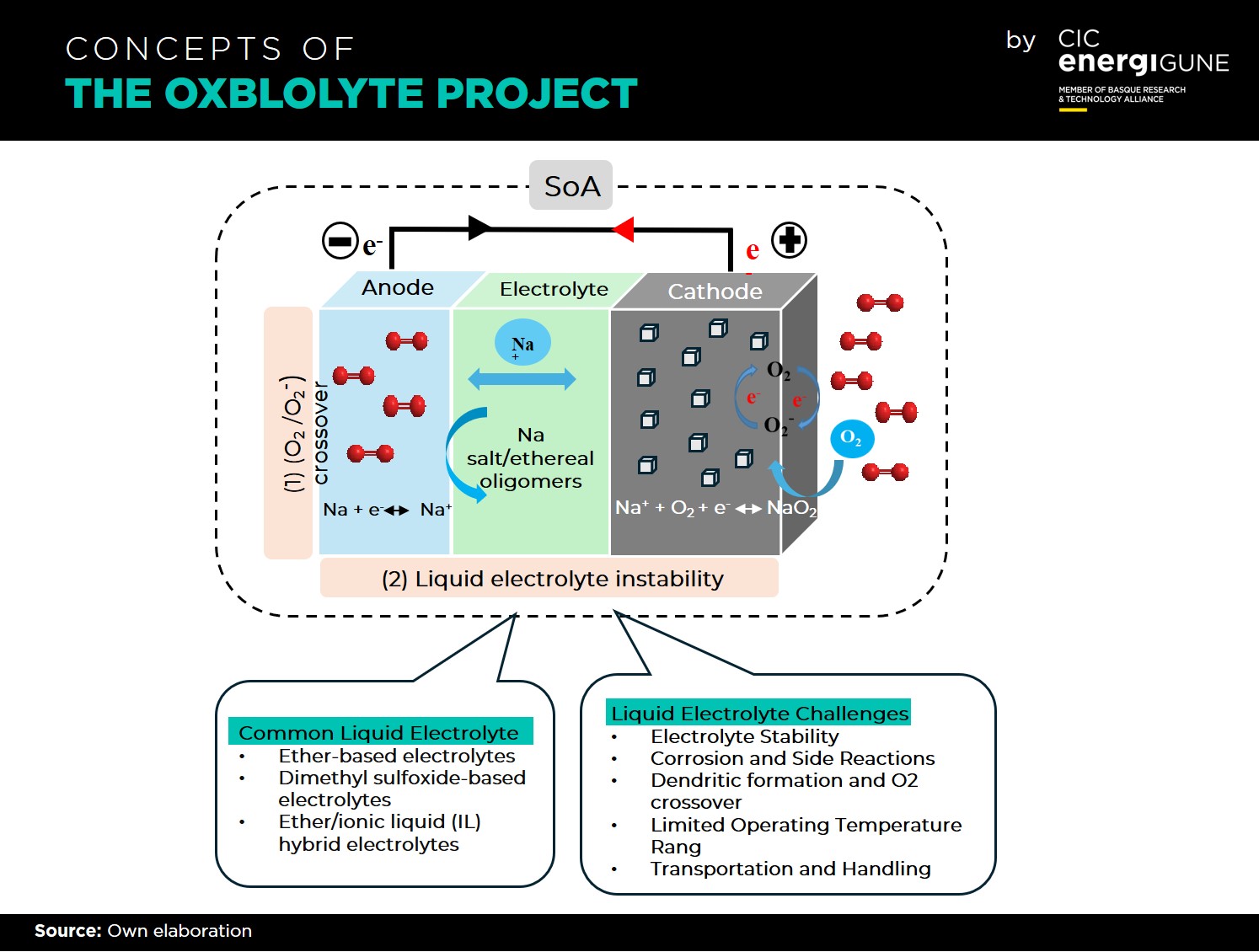
The challenges facing Na-air/O2 batteries are remarkable but not impossible. Issues such as liquid electrolyte instability, O2/O2- crossover, sodium anode passivation, and dendritic growth have been identified and are actively being addressed. Strategies such as exploring solid-state electrolytes (SSE), mitigating O2/O2- crossover, protecting the Na anode, and enhancing the solid electrolyte interphase (SEI) are being pursued as potential solutions.