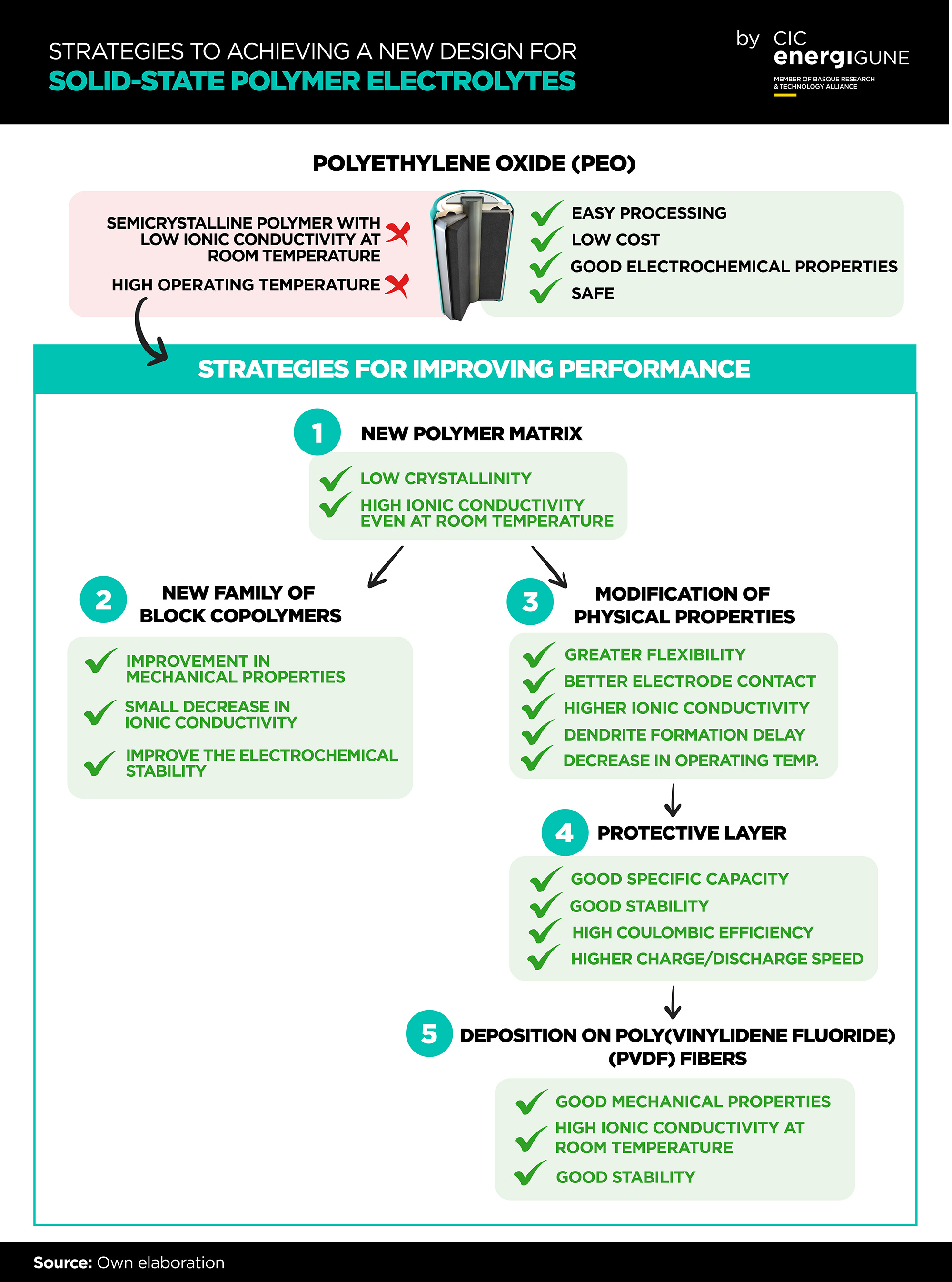
Solid-state polymer electrolytes are good candidates to replace conventional liquid electrolytes in energy storage devices. Polymers have great advantages such as easy processing, low cost, good electrochemical properties and, in addition, they eliminate the safety issues present in liquid electrolytes. However, polymer electrolytes exhibit much lower ionic conductivities than their liquid counterparts at room temperature.
The development of such electrolytes has been hampered by the search for a good compromise between good mechanical properties and high ionic conductivity. In recent years, different polymer structures have been designed with the aim of improving both properties at the same time. However, amorphous solid-state polymer electrolytes with low glass transition temperature and low crystallinity (essential properties to accelerate ionic transport) can hardly form membranes with good mechanical properties.
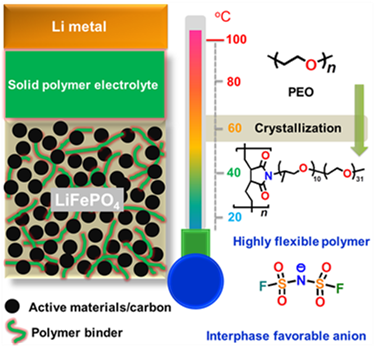
Among all the polymers investigated in the solid state, polyethylene oxide (PEO) has received the most attention. However, this type of electrolyte has several drawbacks. On the one hand, it presents low anodic stability (<4.0 V vs. Li0│Li+). On the other hand, it is a semi-crystalline polymer in which ionic transport occurs mainly in the amorphous phase, leading to low ionic conductivity at temperatures below the melting point (Tm, aprox. 65 oC). For this reason, solid-state batteries based on PEO operate at elevated temperatures (70‒90 oC).
To try to overcome these drawbacks, in recent years CIC energiGUNE has been working on the design and synthesis of non-crystalline polymeric matrices with low glass transition temperatures, good mechanical properties and higher stability against Li anode to avoid the formation of dendrites
Among these modifications at the molecular level, strategies such as the design of comb-like polymeric structures with a high degree of branching, cross-linking of the polymeric network and block or random polymerization can be highlighted.