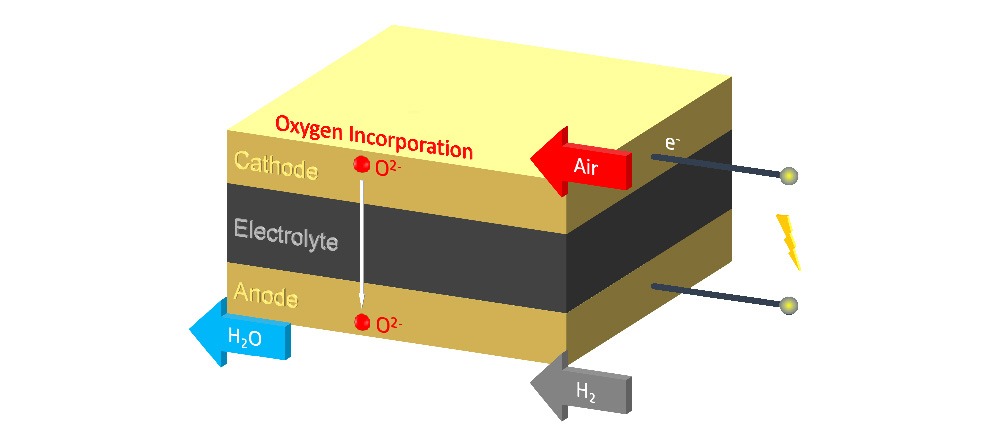
Fuel cells operate by the transport of a mobile ion through an electrolyte separating two chambers with electrodes, as shown in the diagram above. Because the electrons cannot pass through electrolyte they have to pass through the external circuit, generating electrical power from the oxidation of the fuel.
The main advantages of fuel cells are that they have no moving parts, and are thus inherently quiet and efficient, they don’t burn the fuel in a flame as in an internal combustion engine and do not produce particulates or atmospheric pollutants such as NOx, responsible for photochemical smog and consequent health hazards.
The table below lists the main types of fuel cell:
|
Type
|
Electrolyte
|
Fuel
|
Temperature
|
Typical
Application
|
|
Alkaline (AFC)
|
KOH
(liquid)
|
H2
|
50-200 ºC
|
Space vehicles
|
|
Phosporic Acid (PAFC)
|
Phosporic Acid
(liquid)
|
H2
|
~220 ºC
|
Stationary
|
|
Proton Exchange Membrane (PEM)
|
NafionTM
(Polymer)
|
H2
|
70-80 ºC
|
Mobile
|
|
Molten Carbonate (MCFC)
|
Molten
Carbonates
|
H2
Hydrocarbon
|
~650 ºC
|
Stationary
|
|
Solid-Oxide (SOFC)
|
Stabilised Zirconia
Ceramic
|
H2
Hydrocarbon
|
500-1000 ºC
|
Stationary
|
The first three fuel cells, the alkaline, the phosphoric acid and the proton exchange membrane (PEM) are classed as low temperature fuel cells; the two at the bottom of the table, the molten carbonate and the solid oxide fuel cell (SOFC) are classed as high temperature cells and can operate with either hydrogen and hydrocarbon fuels. The most infamous of the fuel cells listed is the alkaline fuel cell used in the Apollo 13 mission where the oxygen tank exploded enroute to the moon.
Each cell of whatever type will only produce a small amount of power, so to achieve kW scale the cells have to be connected together to form a fuel cell stack. In addition, the stack needs an acompanying “balance of plant” to provide gas and air supply and for stack cooling. This all adds to the weight and cost of a finished stack.
Of the 5 listed types of fuel cells the most current interest is being shown in the PEM and the SOFC.
Proton Exchange Membrane (PEM) cells
PEM cells need pure hydrogen as a fuel and are the type of cell stacks most often associated with transport due to their low weight. Examples include the Toyota Mirai fuel cell vehicle and the Ballard fuel cell buses. PEM cells are even being considered for the electrification of short haul air transport. These vehicles have zero carbon emissions at the point of use and release only water as the exhaust, clearly an advantage for urban vehicles to reduce pollution levels.
The hydrogen to power the vehicles is stored in high pressure cylinders. These vehicles are in competition with battery powered vehicles but have some clear advantages, for example the claimed 400 mile (around 650km) range of the Mirai and the rapid recharging compared with a battery powered vehicle.
The main drawback for hydrogen powered cars is the lack of hydrogen recharging infrastructure, set to expand, but which is lagging behind the growing infrastructure for battery powered cars. In addition, converting renewable electricity to hydrogen and then back to electricity in a fuel cell is less efficient than storing the electrical energy in a battery.
The pros and cons of each vehicle type will eventually determine their ultimate fate, but its likely that, at least in the short term, battery vehicles will be favoured for private users and hydrogen fuel cell vehicles favoured by fleet owners.