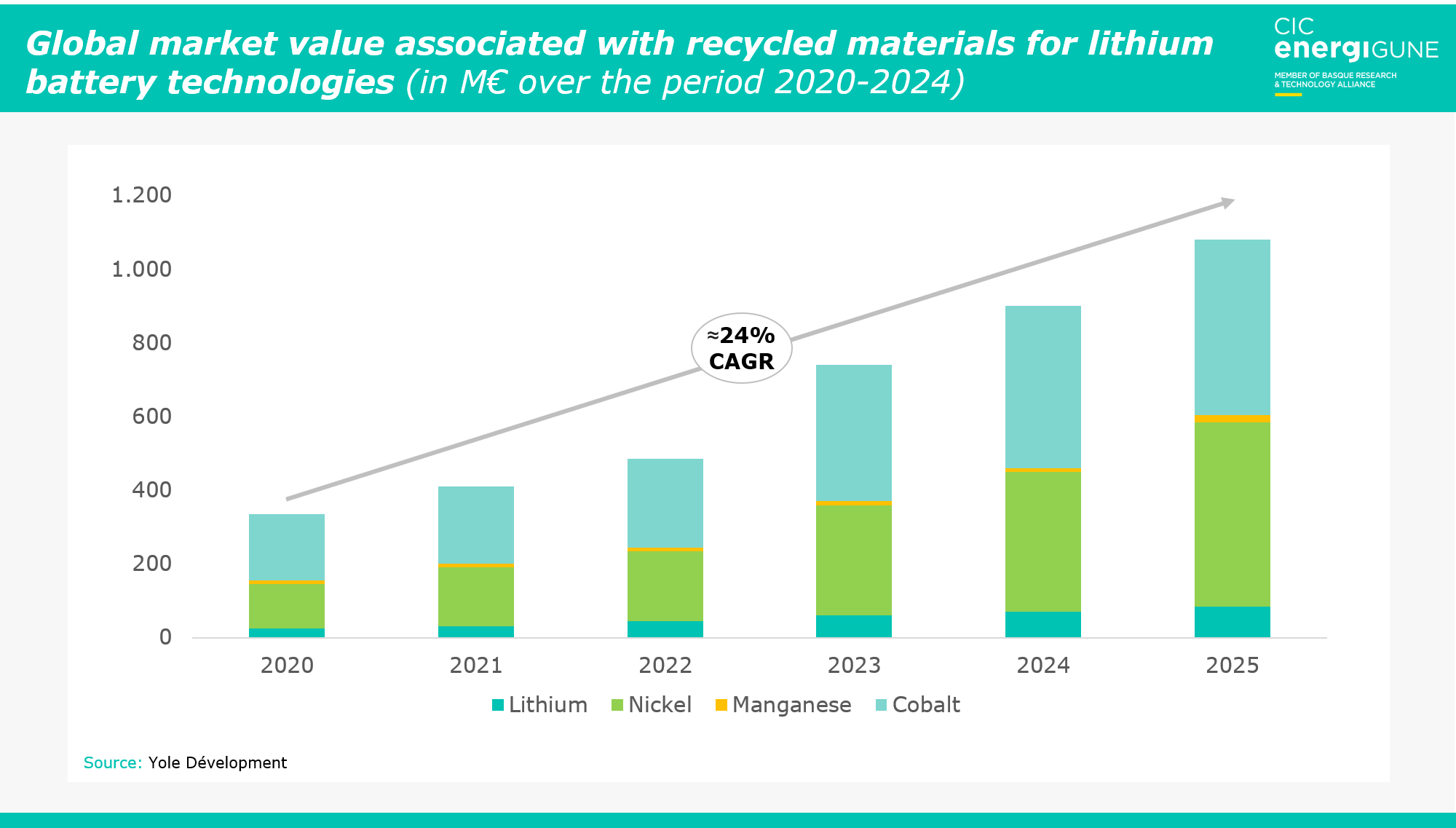
However, in order to achieve these forecasts, it is necessary to develop and improve existing recycling processes, especially in terms not only of the recovery of critical valuable metals, but also of non-metallic elements (such as the battery electrolyte itself).
Therefore, research associated with battery recycling is working to identify new recycling techniques or, if necessary, models and ways to promote and improve existing processes.
The pre-treatment stages as a starting point for proper recovery
The recycling cycle begins with the pretreatment or "preparation" of the batteries to be recycled.
This preliminary stage is critical for the progress of the recycling procedures, since in many cases the safety of the entire subsequent procedure depends on it, as well as the possibilities of recovering the greatest number of battery components.
Therefore, through this first step of the procedure, the aim is to ensure the correct disassembly of the battery (ensuring a safe handling of it) and the efficient separation of the components to be recovered later on.
There are two main pretreatment alternatives:
The first one is mechanical pretreatment, consisting of discharging, grinding and separating the battery elements through a mechanical-based technology (generally magnetic, pneumatic and/or gravimetric separation).
This is one of the most effective formulas for pretreatment (especially in relation to hydrometallurgical techniques, which will be detailed below), even more so in recent years thanks to new automation and artificial intelligence solutions that have made it possible to digitize the process.
The other major alternative that has been gaining importance in recent years is thermal pretreatment, which, as its name suggests, is based on heat methods for the development of the process.
Thus, it is carried out through distillation, pyrolysis, thermolysis or combustion operations to eliminate/recover organic components, resulting in safer handling of battery waste, and also improving the chances of recovering the electrolyte and binder (although in the latter case, it depends on the type of the electrolyte and the technique used). Due to its nature, it is usually part of the pyrometallurgical techniques that we will see below, although it can be perfectly possible to use it with other routes.
The promising hydrometallurgical route
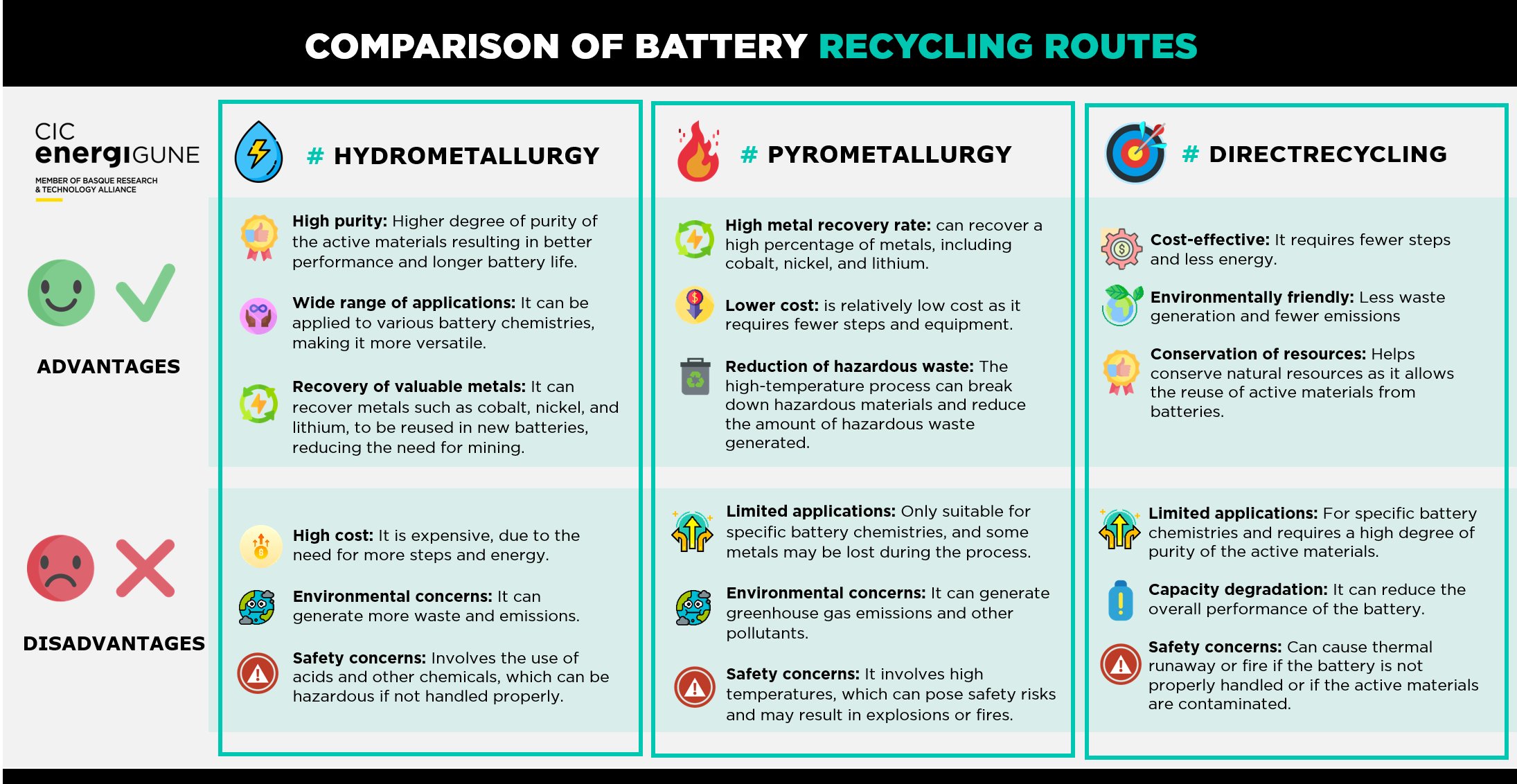
Moving on to the recycling process as such, it seems that the hydrometallurgical route is the option most likely to take over the battery recycling industry in the future thanks to the current results and forecasts for improvement (both for current technologies and potential new solutions). In fact, this option is considered to be the most promising if the future European Directive on Batteries is taken into account.
Hydrometallurgical techniques rely on the acid solubility of the elements present in the active materials (organic or inorganic) to carry out the recovery of these materials by dissolution.
This is a process with several stages, including pretreatment (to ensure the safe handling of the batteries, generally based on the aforementioned mechanical methods); leaching, which achieves the recovery of the active materials; purification, which allows the elimination of accompanying substances that limit the necessary quality of the product required; and finishing (electrolysis, crystallization, coprecipitation...), which produces the precursors of active material to manufacture new batteries.
The great advantage of this technique is the high recovery of several components, recovering even the existing graphite. Moreover, all of this is of a high enough quality to be reused again by the battery industry (known as "battery grade" products), thus promoting the circular economy.
Although electrolyte recovery is not currently being achieved, the first studies are under way and, with somewhat more complex processes (in combination with thermal pre-treatment), are making progress in the recovery of electrolyte through these hydrometallurgical techniques.
Moreover, the fact that it does not produce toxic gas emissions during start-up makes it one of the most attractive options for the development of the recycling industry. Even more so if it is possible to reduce the generation of wastewater in the coming years, something which, by its very nature, this process entails.
The pyrometallurgical alternative
Alongside the hydrometallurgical route, pyrometallurgical solutions have shown the best results to date.
These techniques base their procedure on the use of high temperatures (around 1,500 ºC) to melt the batteries and thus burn all the carbon-based compounds.
In this way, the valuable metals included in the battery end up in a resulting alloy (which, depending on the composition of the battery, can be more or less rich in cobalt, nickel or manganese), that can then be recovered individually through dissolution by means of hydrometallurgical processes.
In addition, and in contrast to the hydrometallurgical route, this alternative reduces the previous handling and pretreatment phases of the used batteries, thus "speeding up" the recycling process. This, together with the ease of controlling the risk of fire and explosion during the process, makes this route one of the safest options for implementation.
However, the fact that heat is used as the basis of the process is the biggest disadvantage of this solution. As a result, it is very difficult to recover many of the battery components, such as electrolyte, graphite, steel, aluminum or lithium, as they are all lost as slag or "off-gas".
In addition, as mentioned above, the individual recovery of other components such as existing metals may depend on the use of hydrometallurgy techniques, which in many cases may mean that the latter alternative is chosen in its complete cycle; even more considering that the hydrometallurgical route presents a lower atmospheric impact than the pyrometallurgical one, since in the latter case there is a high volume of atmospheric emissions due to the use of high temperatures as the core of the process.
New tecniques and challenges on the horizon
Both the hydrometallurgical and pyrometallurgical routes are the alternatives that currently have the greatest potential and on which the sector is working to achieve their optimization and improvement.
However, due to the difficulty of minimizing some of the weak points of each of these alternatives, in the last few years, other options are being worked on to try to find a new alternative that improves the current results.
In this way, the so-called "direct recycling" is the one that has recently received most attention as a potential "third way" to the existing ones. The basis of this process is simple: the aim is to recycle the battery while leaving the crystalline structure of the active cathode material intact.
Thus, through direct recycling, the aim is to restore the initial properties and electrochemical capacity of the cathode materials without decomposing into substitute elements, which can be directly reused for the manufacture of new batteries. To this end, different mechanical, thermal, chemical and electrochemical processes are being analyzed to "revive" the battery during the recycling process.
The objective, after all, is to optimize the recycling process in order to achieve the highest profitability and minimize negative impact both in terms of recovery and environmental impact.
However, this optimization and improvement of the processes (both the most established ones and the new ones under development) not only seeks to promote the current state of the art, but also to prepare the new battery technologies that are foreseen for the future.
Therefore, the medium-term research focus is on the development of new hydrometallurgical formulas or incipient processes such as direct recycling, in order to meet the challenges of the massive adoption of solid state in the future if the forecasts are fulfilled.
Until that moment, and as a summary, at CIC energiGUNE, we provide below a brief infographic of the current situation of the most established recycling processes, with the aim of being able to make a quick comparison between their advantages and disadvantages: