In recent decades, materials science has provided us with true prodigies with new, improved and even combined properties to respond to the challenges we face in research, which also facilitate industrial processes or our lifestyle.
In this knowledge area, we have been talking about advanced materials, those with improved thermal, chemical and/or mechanical properties (among others), and even those designed specifically for a particular application. Among these properties, there is one that often goes unnoticed by the general public, but which is crucial for countless applications, especially for those related to energy generation and storage: porosity.
When we talk about porous materials, we might think of a sponge or a pumice stone, both porous, natural and with applications that are directly conditioned by their porous nature. The sponge can hold large volumes of liquids in its cavities, and the roughness of the pumice stone, generated by its pores, makes it an excellent abrasive for our skin. However, the pore size of these materials is in the millimeter, even centimeter range.
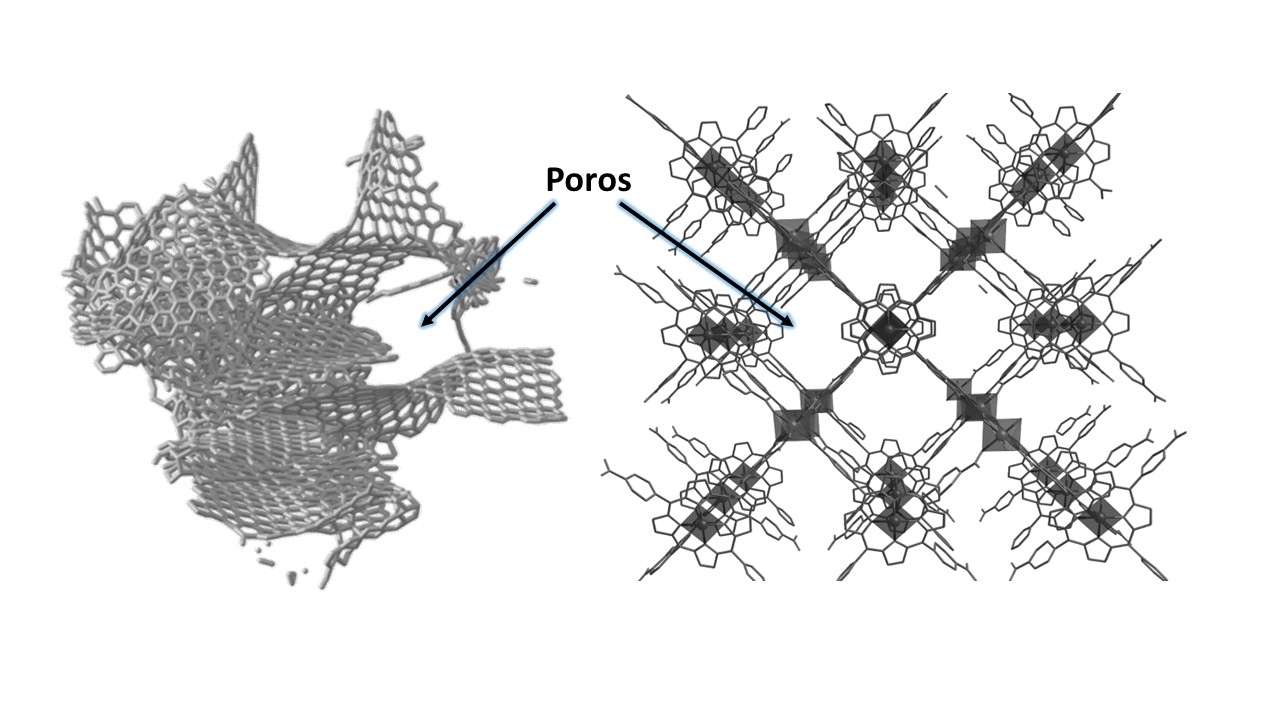
On this occasion, we are interested in pores that are on the nanometer scale, which is about six orders of magnitude less than the previous examples. As in other areas of knowledge, going down to the nanometer can enhance the properties we wish to exploit.
Aspects like pore volume, pore size and surface area define the texture of a solid. It is in this empty space within the material and on its inner surface that a wide variety of physical and chemical processes can take place. That is why it is important to control these properties, to get the most out of them in our applications.
A simple analogy will help us to understand this. If I need to store a substance on the surface of a material (what we know as the adsorption phenomenon), I could use an empty vessel, in which the accessible surface for the substance to be stored would be the vessel´s own inner wall. Let us assume, in this case, a few tens of cm2 in a bottle of 1 L volume. Now, if I fill that same bottle with a porous material with an internal surface area of about 300 m2 for each gram of material, I would be substantially increasing the surface area accessible to the substance I want to store on the surface: the tens of cm2 of the container wall plus hundreds of m2 for each gram of material I introduce in that container. The storage efficiency in the latter case is evident.
This happens thanks to the nanopores present in the porous solid, which increase the accessible area where the host molecules that we want to store or transport are located, where the desired chemical reactions occur or where charge transfers take place, among other possible phenomena.
Amorphous or crystalline porous materials
Historically, the most commonly used porous materials have been activated carbons or zeolites. The main difference between them is that the former are amorphous (their atoms are randomly disposed of in their structure) and, therefore, the porosity does not have an ordered layout. The latter are crystalline (their atoms and molecules are oriented in their structure based on a certain symmetry), which gives them an ordered porosity and a specific topology.