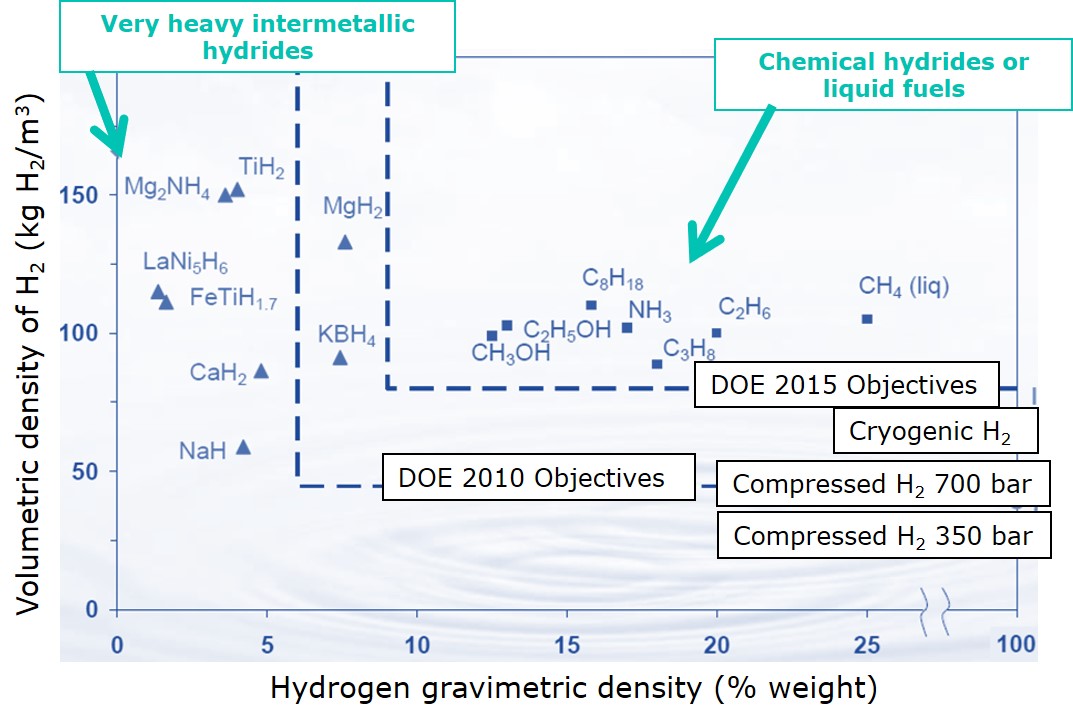
Among the non-organic-hydrogen-containing-liquid-fuels, ammonia (NH3) is the top candidate. It contains 17% hydrogen by weight, which can be extracted via thermal catalytic decomposition or via electro-oxidation. Alternatively, NH3 can be potentially oxidized directly in fuel cells without the need for a separate reactor.
The energy density of NH3 (12.7 MJ/L) is even higher than the energy density for liquid hydrogen (8.5 MJ/L). Moreover, ammonia can be stored at a much less energy-intensive –33 °C, than the –253 °C of cryogenic stored liquid hydrogen. Besides, ammonia is also less flammable than hydrogen. Finally, because 200 million metric tons of NH3 is already produced annually, a vast infrastructure for storage and transportation of ammonia already in exists.
Currently, this amount of ammonia is produced primary through the energy intensive 100-year-old Haber-Bosch process which is not compatible with the energy transition because uses H2 from thermal cracking of methane and high synthesis temperatures.
However, new greener alternatives that will enable the use of ammonia as a liquid energy vector are surging. As an example, and probably the most ambitious project, is the ammonia synthesis plant to be located on the Red Sea coast of Saudi Arabia. A photovoltaic plant will harness the sun during the day, while turbines will capture night-time winds, to generate 4 GW of electricity for water electrolysis plants. The hydrogen will be then fed into a traditional Haber-Bosch plant to produce 1.2 million tons per year of NH3.
Yara, one of the major producers of ammonia in the world, is planning to produce 75,000 t of NH3 a year, at its ammonia plant in Sluiskil (The Netherlands), using hydrogen from water electrolysis. These electrolyzers would run on 100 MW of power from a new offshore wind farm.
Today, ammonia represents the best option for long-haul transportation; in particular, for trains and ships. An example of this is the EU funded project ShipFC, aimed at developing, installing and testing long-distance-vessel powered by ammonia fuel cells.
At the CIC energiGUNE, our researchers are pioneers in the development of new materials and technologies for the production and utilization of green-hydrogen-containing-liquid fuels.
Hydrogen hydrides: the energy powder
Metal hydrides are an alternative way to store hydrogen at low pressures in a solid. The hydrogen storage at low pressure is feasible because the hydrogen molecules are chemically bonded within the metal compound structure. Metal hydride storage systems typically operate at 10-40 bars, which is 20 times lower than typical high-pressure hydrogen storage systems.
The sizing of the metal hydride storage systems is determined by the specific application needed.
However, one of the main drawbacks of metal hydrides is the weight. The storage capacity of the metal hydride storage is around 1.5kg of H2 (or 50 kWh) per 100 kg of the metal hydride compound material. Even though this value of the energy storage capacity of the metal hydrides is low in comparison with other hydrogen storage systems, it is comparable to the energy capacity of a standard Lithium-ion battery in a Tesla Model 3 (50 kWh).
The other inconvenience of the metal hydrides is the complexity of the system for storage and utilization. First, the hydrides must be stored under nitrogen or argon and protected from water. Secondly, while liquid fuels such as alcohols or ammonia, can be used directly as fuels in fuel cells, the metal hydrides require an activation step for the release of the hydrogen from the structure. When the hydrogen is needed, the desorption of the hydrogen is promoted by a heating step (50 – 100°C). This temperature-driven desorption step is inconvenient for automotive application, in particular during acceleration and deacceleration steps.
Other alternatives to the temperature-driven desorption include the activation by contact of the hydride with water moisture. In this case, when hydrogen is required, the hydrate is mixed with controlled-humidity air, and the resulting reaction produces high-purity hydrogen. Although the release kinetics are fast, this reaction requires water to be carried onboard separately, adding weight and complexity to the transport application.
Even though, the utilization of metal hydrides for hydrogen storage in transportation is challenging, it is not impossible and the metal hydrates are taking their space across stationary storage and portable electronics. For example, Hydrostik is a convenient hydrogen storage solution to fuel your hydrogen powered devices. GKN hydrogen has deployed different projects that demonstrate the feasibility of short-term and long-term hydrogen storage on metal hydrides for stationary applications.
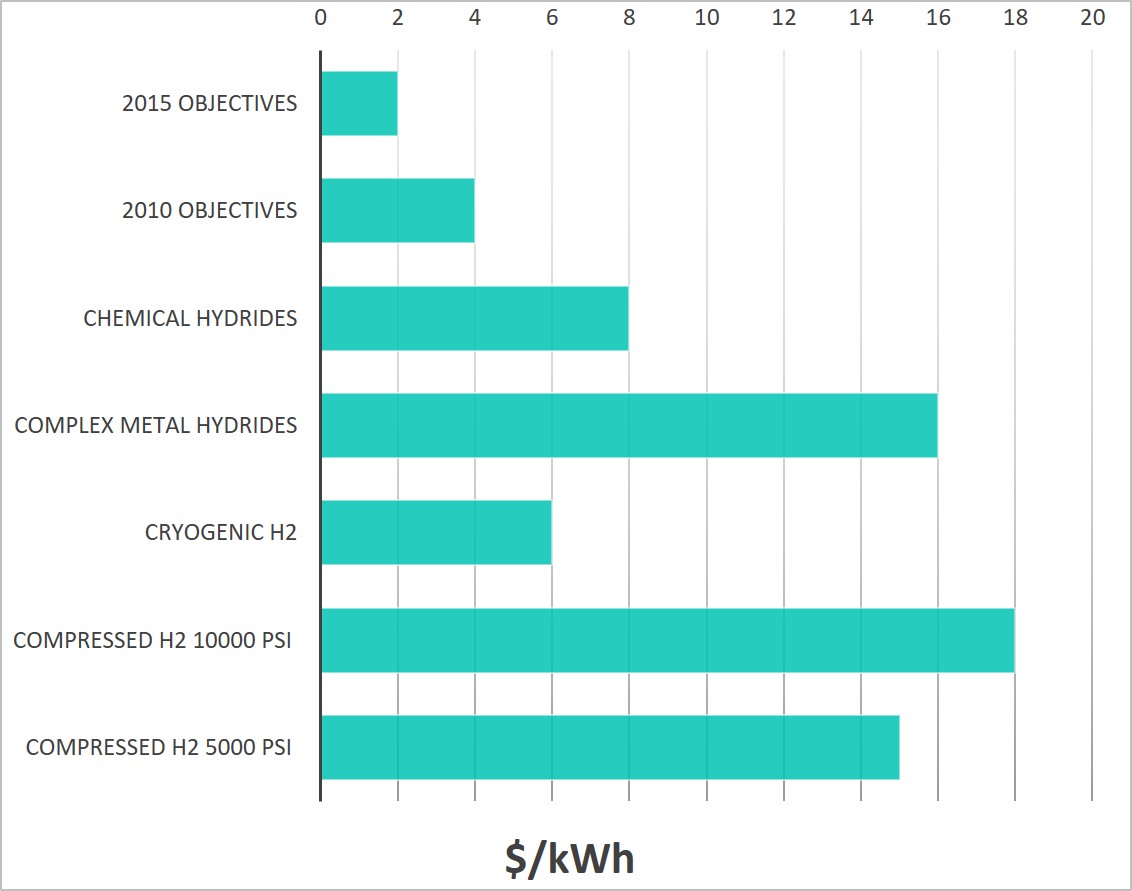
Beyond the technical challenges, currently the hydrogen storage methods do not meet the cost targets proposed by the USA Department of Energy (DOE). Reducing the cost of energy storage is essential to the full deployment of hydrogen economy. Some of the cost challenges will be addressed by exploring new materials or by developing more efficient synthesis process. Furthermore, scaling up and mass production of these technologies will significantly contribute to the reduction of the cost.