Moreover, this attractive energy storage technology employs abundant and environmentally-friendly materials (i.e., is eco-friendly), has great potential for high recyclability, and is safe (i.e., aqueous electrolyte, no need of hazardous organic solvents). As a core battery material, zinc is attractive because it is plentiful, geographically dispersed and much less costly than lithium.
But are these batteries new? Not at all, primary Zn-air batteries are a century old technology whose development is a story of serendipity.
In 1868, Leclanché invented the first cell containing amalgamated Zn anode, a mixture of manganese dioxide and carbon cathode, and ammonium chloride electrolyte. Leclanché unknowingly exposed the cathode to air creating the so-call three-phase boundaries (gas, liquid and solid) and therefore he unintentionally created the first primary Zn-air battery!
Since then, many others (e.g., Maiche, Walker and Wilkins) continued developing better cell designs and materials until 1932 when the first batteries were commercialized by Union Carbide Company in the United States. The primary Zn-air batteries became ubiquitous and nowadays we can find them in hearing aids, patient monitors, road traffic signaling, among others.
In the last years, Zn-air batteries have been revisited with the focus on making them rechargeable due to their promising properties: large theoretical energy density (1353 W h kg−1 excluding oxygen), low cost (currently <$100 kW−1 h−1, and potentially <$10 kW−1 h−1) and inherent safety.
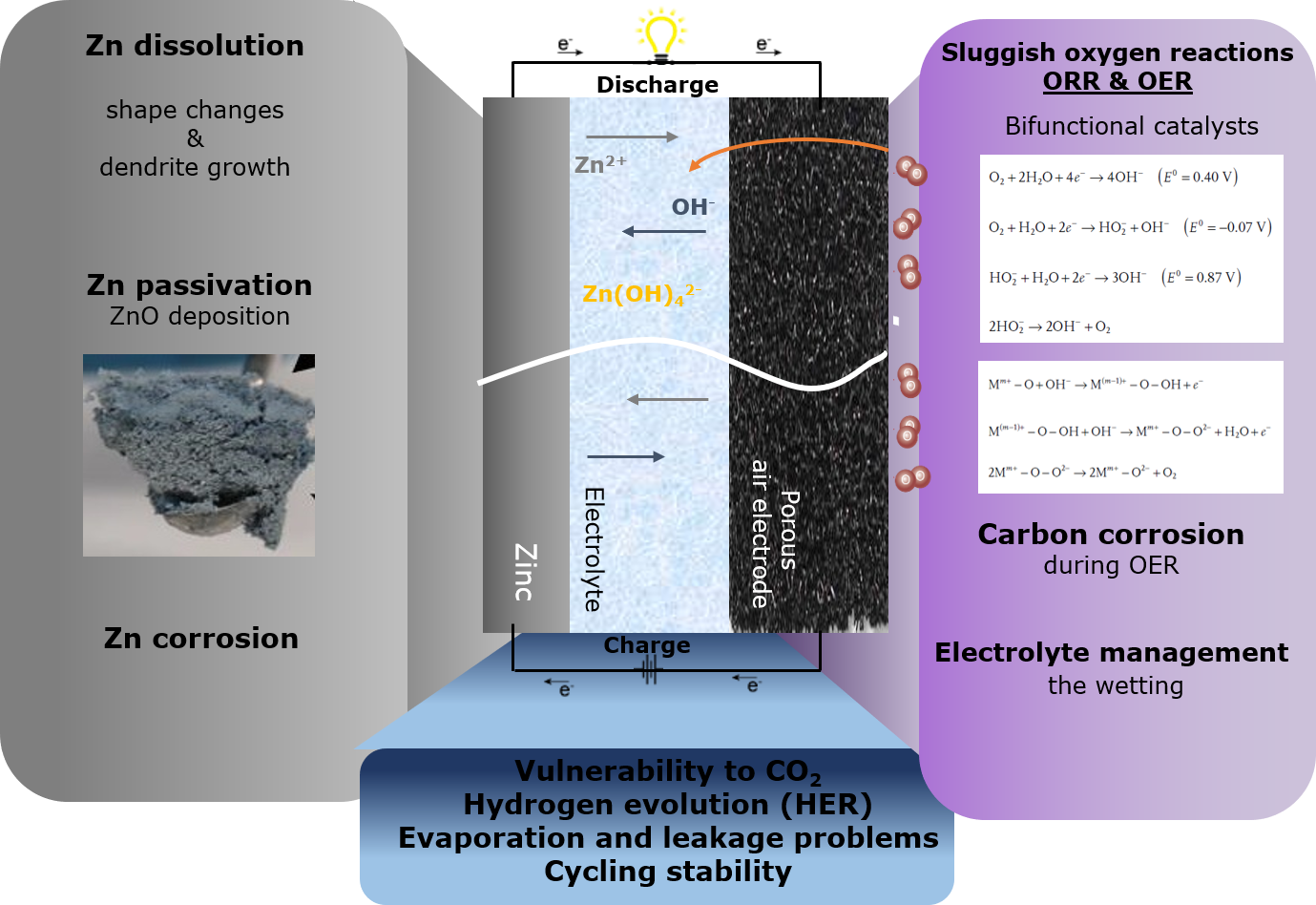
A typical Zn–air battery consists of a porous air cathode and a Zn metal anode, separated by an alkaline electrolyte. During discharge, O2 from the air permeates the porous cathode and gets reduced on the surface; meanwhile, the metallic Zn anode is oxidized to soluble zincate (Zn(OH)42−) ions. Theoretically, this reaction can be reversed with O2 evolving at the cathode and further released back to the atmosphere, and metallic Zn plated at the anode. These operating principles are based on the oxygen reduction reaction (ORR) during discharge and evolution reaction (OER) during charge.
One of the major challenges of this energy storage technology is the sluggish kinetics of these reactions which lead to poor cycle life. Worldwide researchers have therefore oriented their investigations towards developing proper bifunctional oxygen electrocatalysts (active during ORR and OER).
At the current state of technology, precious metal or transition metal oxides have been studied and developed as the best performing bifunctional catalysts; however, the use of cheap and conductive carbon material supports improve the catalyst dispersion and active area while decreasing the cost of the final catalyst. The inherent advantage of these batteries is that they do not require any critical raw materials and the carbon-based materials can be, for example, used from the foundry waste aiming at a circular economy.
Far less attention has been paid to the Zn anode which is equally important to achieve stable and cyclable Zn-air batteries. The main challenge arises from the spontaneous reaction between Zn and the electrolyte which generates H2 and electrode corrosion, leading to cell failure. Current studies on rechargeable Zn-air batteries are far away from practical applications; the depth of discharge (DOD) is mostly very low (<1%) which barely challenges Zn anodes. Commercial applications require high Zn anode utilization (>50% DoD) and electrochemical reversibility under practical circumstances. In this sense, research and industry need to work together to find sustainable, lasting solutions to develop a rechargeable Zn-air battery.
In the last years, several companies (e.g., NantEnergy in US, EDF in Europe) have claimed the development of electrically rechargeable batteries with no hazardous substances, inexpensive and fully recyclable. However, these breakthroughs are surrounded by a halo of skepticism.
It will be a matter of time until these batteries are release to the general market. A market with plenty of opportunities as Li-ion batteries are falling short as a storage solution for balancing the supply and demand of electricity of the power grid and for avoiding costly peak-time charges.
The interest on rechargeable Zn-air batteries is continuously increasing because their mass production is feasible at cheap prices. For example, for large-scale energy storage, Li-ion cannot compete with Zn-air batteries.
The future looks promising and the effort dedicated to the development of this energy storage system increases. We could consider it as the holy-grain of batteries: a battery made of abundant and environmentally materials, safe, cheap and recyclable.
Now is the time for scientists (metal-air research line at CIC energiGUNE) and companies to work together to make this technology a reality. We need to store more and more energy and this system has numerous advantages for stationary applications where cost is a must.

Author: Nagore Ortiz Vitoriano, Metal-air batteries research line manager of CIC energiGUNE.

If you want to know the latest trends in energy storage and new developments in research, subscribe.

If you want to join a top-level team, collaborate with specialists in multiple disciplines or tell us about your concerns, don't think twice...