Lithium-ion batteries are nowadays mainstream for many applications and have already transformed our society by enabling the wireless revolution. But the challenge today, with the electric vehicle industry in expansion, is massive, as our community must evolve towards full decarbonization.
Unfortunately, Lithium-ion batteries will be unable to meet demands such as higher energy density and improved lifespan solely on their own, which is why the energy storage sector is already taking a step ahead.
New terms that might at first result confusing, are thus increasingly found in scientific literature and media: post-Li-ion, post-Li, beyond Li-ion, beyond Li, beyond Lithium, etc.
All these are umbrella terms covering different technologies: Sodium-ion (Na-ion), Zinc-ion (Zn-ion), metal-sulfur (Na-S, Li-S), metal-air (Na-air, Li-air), solid-state batteries, redox-flow, etc. These differ in key components and redox chemistry, resulting in separate challenges, metrics, and maturity.
In order to understand the main differences between all the rising electrochemical energy storage technologies we will clarify some terms and concepts.
Battery technologies boom
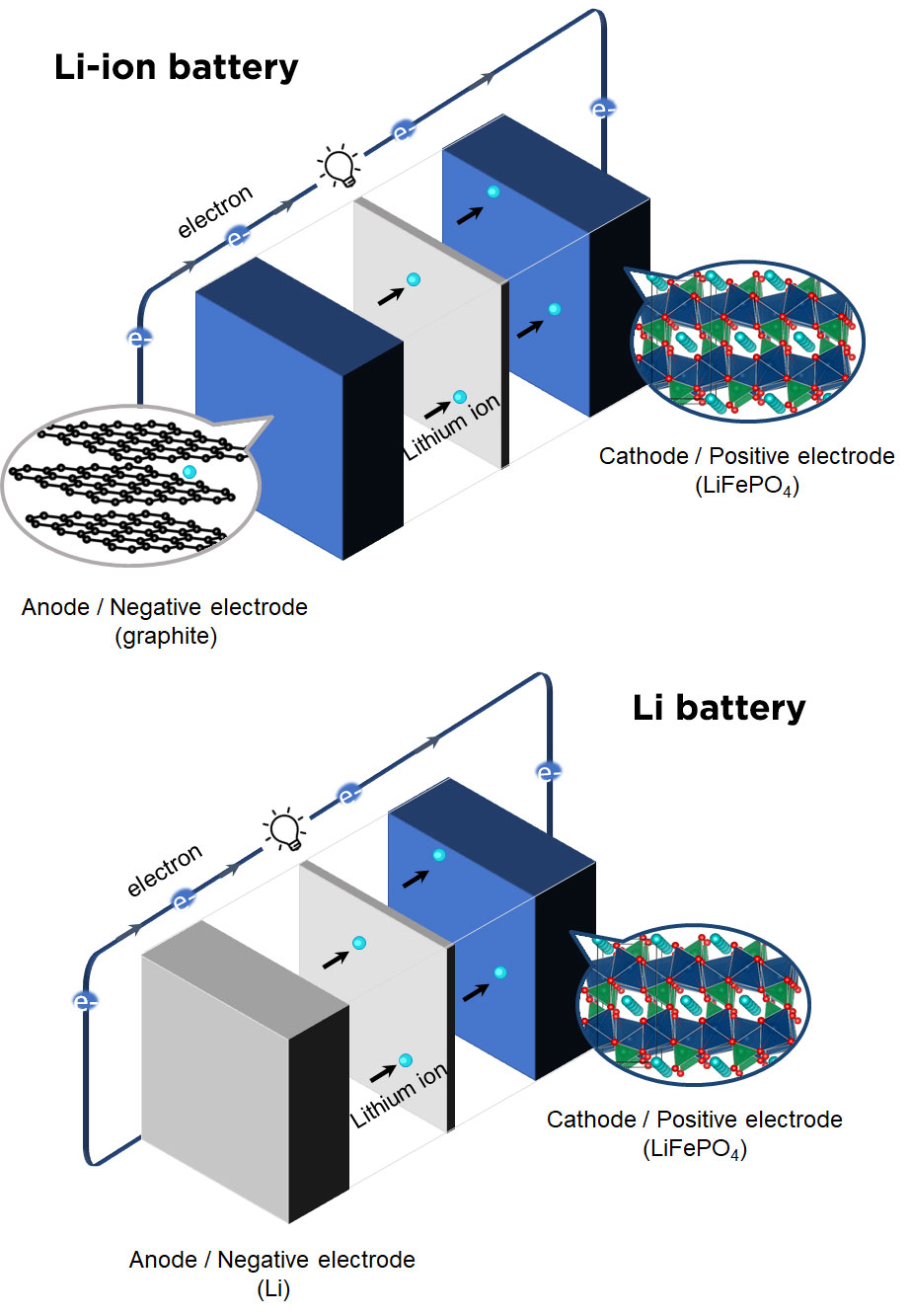
Batteries store electrical energy through redox reactions. When the battery discharges, chemical species at the negative electrode are oxidized, releasing electrons that provide electrical current and are consumed by the chemical species at the positive electrode.
For every electron that goes through the external circuit, an equivalent amount of positive charge, carried in the form of ions, is also consumed in order to balance charges. In Li-ion batteries, the charge carriers are Li+ cations, and these are shuttled between the two electrodes that must be able to accommodate them (for example, NMC or LFP and graphite).
Metal-ion batteries (Li-ion, Na-ion, K-ion, Zn-ion, Mg-ion, Ca-ion, Al-ion) differ in the charge carrier. The higher the charge carried by an ion, the higher the resulting specific capacity, but the more challenging the migration of multivalent ions within electrolyte and electrodes.
On the other hand, if a metal anode is directly used (instead of a host material able to accommodate the alkali ions), we’ll drop the “-ion” from the generic term and speak about a (rechargeable) metal battery. For example, in a Lithium battery, metallic lithium replaces graphite, resulting in much larger capacity but also important safety issues that still need to be solved.
Note that Li metal anodes were retired from the market in the late 80s, but intensive research efforts are currently ongoing for a potential coming back. Likewise, other charge carriers could be potentially used, leading to sodium, magnesium, or calcium batteries, for example.
Therefore, post-Li-ion batteries (or beyond Li-ion) enclose all kinds of emerging storage technologies that represent a step forward concerning current Li-ion batteries (including Li metal batteries).
On the other hand, post-Li batteries (or beyond Li batteries), necessarily involve a different charge carrier other than Li+.
In both cases (post-Li-ion or post-Li) different concepts exist.