However, both scientific and technological challenges are still to be solved to make thermochemical cycles competitive.
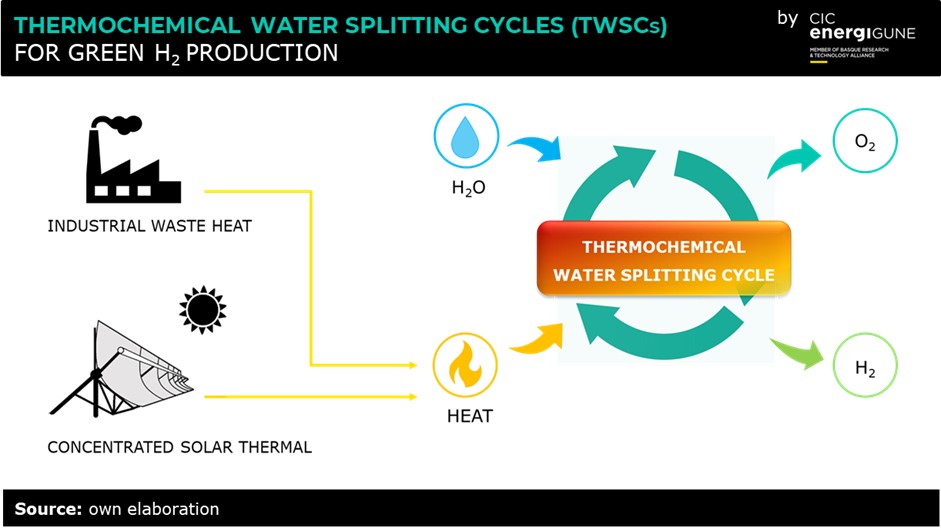
The research performed at CIC energiGUNE aims to develop decentralized hydrogen production modules to be integrated, for example, in industrial environments with waste heat as a heat source.
But let us start from the basics.
Thermochemical water splitting: old but gold?
Water thermolysis is like water electrolysis but using heat instead of electricity.
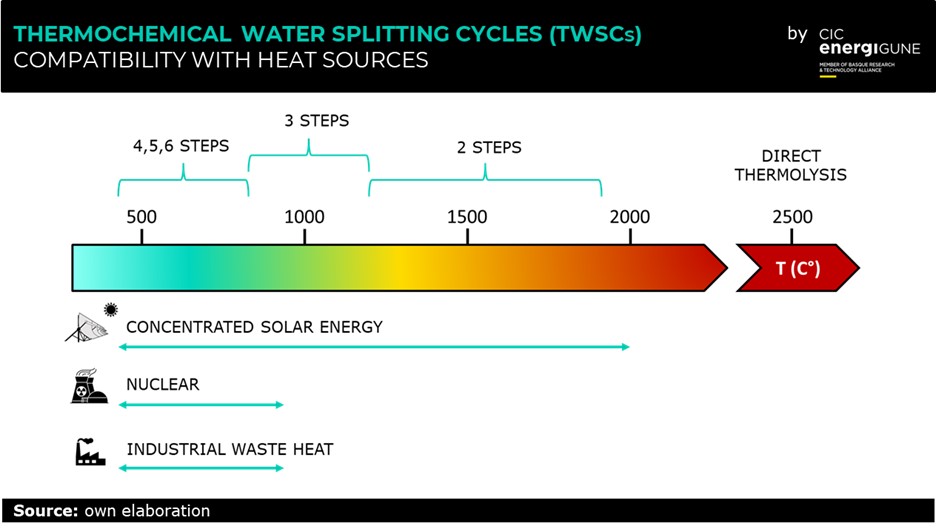
We all know that the chemical formula of water is H2O, right? Well, in principle, if we simply heat up enough, we can split water into hydrogen (H2) and oxygen (O2). However, such direct water thermolysis requires temperatures >2500ºC, which makes it unsuitable for real-life applications.
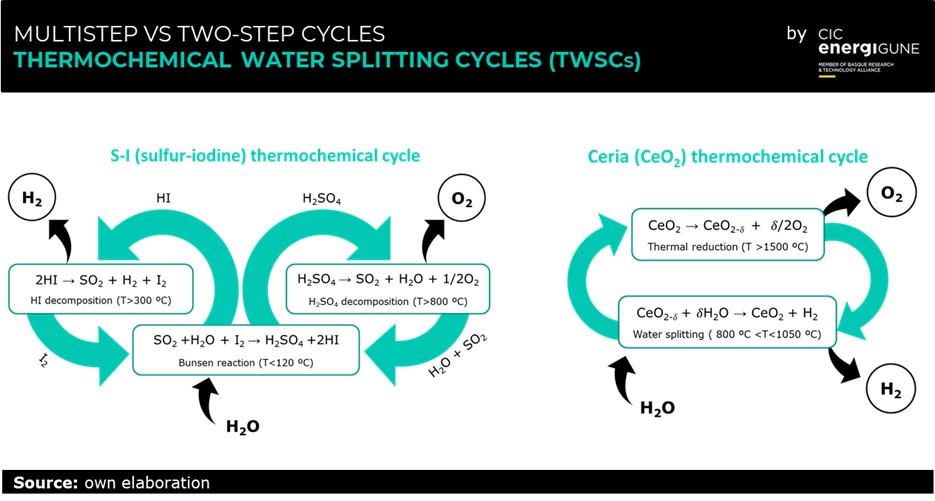
This main problem is solved using a certain number of chemical reactions to divide the water decompositions into more steps. The reactants are regenerated in a closed loop while the overall chemical reaction remains that of direct water thermolysis. The result is what is commonly called as thermochemical water splitting cycle (TWSC). Thanks to TWSCs operating temperature decreases, and hydrogen and oxygen are produced in different steps, which eliminates the need for high-temperature gas separation and reduces the risk of accidents.
This trick is anything but new.
The idea behind TWSCs dates to 1966. The interest in this technology then increased during the 70´s and the 80´s due to the oil crisis. At that time, a huge amount of TWSCs was proposed with the main idea of producing hydrogen from nuclear energy.
Since their conception, TWSCs have been considered the natural alternative to water electrolysis for large-scale H2 production. One of the main advantages presented by TWCSs is that they are catalyst-free and do not rely on expensive and rare elements.
The research on TWSCs boomed at the beginning of the 21st century when climate change awareness grew rapidly among the scientific community. Nowadays, the technological advances achieved in solar energy collectors and concentrators make coupling TWSCs with concentrated solar energy particularly interesting for green hydrogen production. Moreover, the utilization of waste heat has great potential for decentralized hydrogen production in proximity of industries or power plants. Creating TWSCs modules could help to decrease the overall CO2 footprint of the industrial process and find application in smart grids, together with electrolyzes and thermal energy storage (TES) units.