Which polymers are competitive candidates to be used as electrolytes?
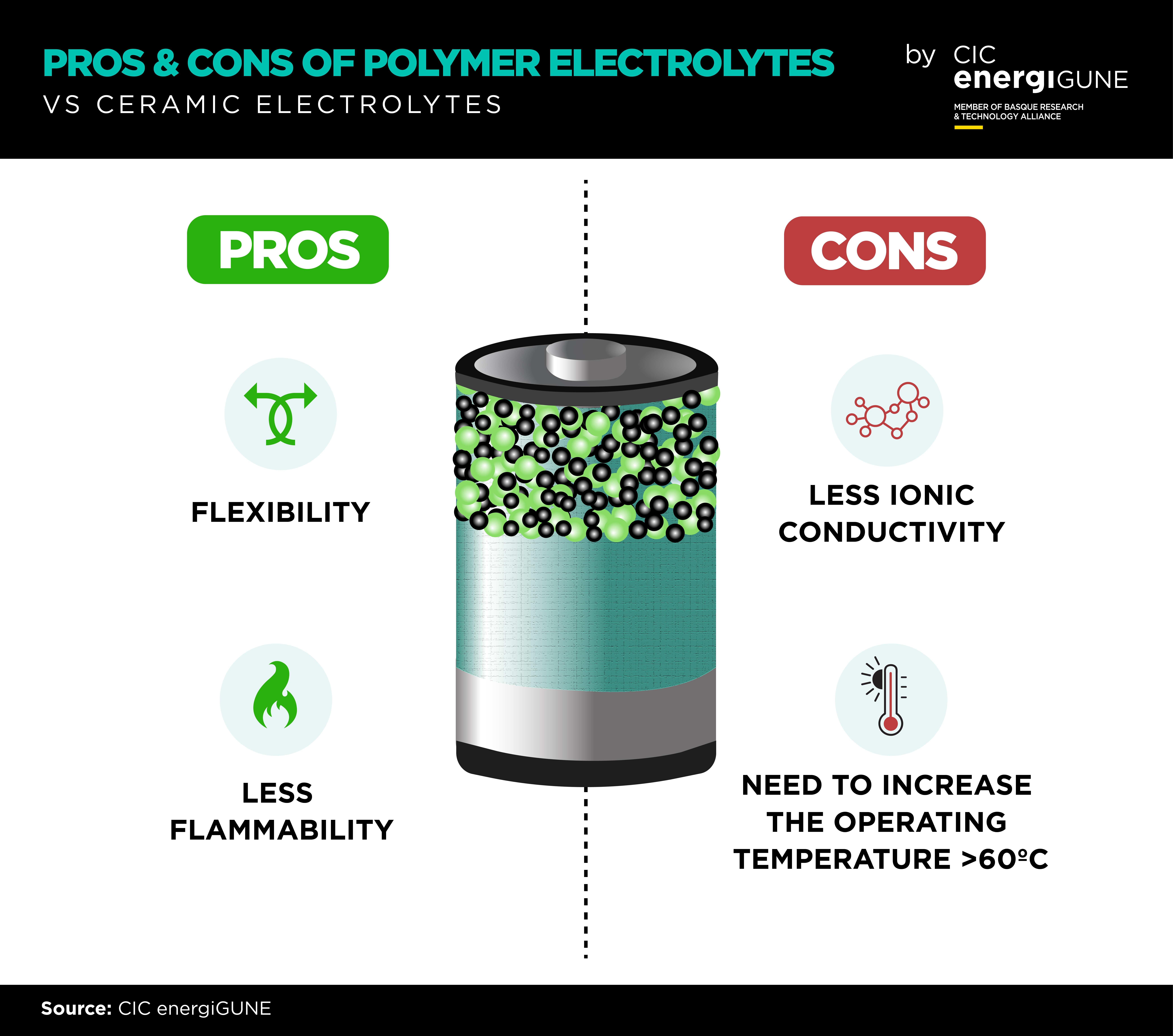
The electrochemical properties of polymer electrolytes are related to the thermal properties of the polymer. Among these properties, an amorphous and flexible polymer with high molecular weight and polar chemical groups is sought. Historically, different polymers have been proposed for their application in batteries, many of which are used in our daily life.
- Poly(ethylene oxide) (PEO) is a biocompatible and non-toxic material whose low molecular weight counterpart is used in biomedicine.
- Poly(methyl methacrylate) (PMMA) is a hard, scratch-resistant material that is widely used in medicine, e.g. for bone and dental prostheses, and in various coatings.
- Polycarbonate (PC), being a non-toxic material, can be found in many applications: panels, industrial machinery, or technological shields.
- Polyacrylonitrile (PAN) with high thermal stability, strength and modulus of elasticity is employed for the manufacture of synthetic fibres, known as acrylics.
- Polyethylene (PE), which offers good heat and chemical resistance, is used in food packaging, stretch film and tubing for cosmetics and pharmaceuticals.
- Polyvinylidene fluoride (PVDF) is chemical and temperature resistant and can be found in chemical tank linings.
The application of these polymers in batteries has required adapting their chemistry to meet the demands of such devices. In particular, to improve the segmental motion of the chains by replacing rigid groups with more flexible ones.
Among the proposed polymers, PEO was suggested in the 1980s and is currently the reference material in this field. It is compatible with lithium metal negative electrode and provides good conductivity at 70 °C. However, it is observed that the anion moves faster than the cation, causing polarisation during cycling. In addition, ethylene oxide units tend to oxidise at high voltage, leading to poor voltage stability and limiting their application in contact with high voltage active materials. However, it is noteworthy that it is the only polymer chemistry used as a lithium-ion conducting matrix in a commercial battery (technology developed by BlueSolutions).
The conductivity of lithium ions is significantly improved with the polymeric matrix of PC and PAN. These polymers also offer high voltage stability, allowing the application with high voltage active materials (i.e. NMC811). Nevertheless, the instability against lithium metal limits the use in contact with the negative electrode and suggests its application exclusively in contact with high voltage materials. This indicates another polymer chemistry should be proposed as an electrolyte matrix.
In most cases, polymers offer high ionic conductivity when viscosity decreases. This implies that the mechanical properties must be improved to avoid short circuiting. When polymers of different properties are blended, one of them will provide the conductivity, while the other will ensure the mechanical integrity; this is the role of PE and PVDF.
Still the polymers have to face the challenge of the automotive industry requirements, especially when it comes to ambient temperature applications.
In which direction are we going?
In order to reach the requirements set by the automotive industry, adjusting the thermal properties of polymers may be an option: decreasing of the crystallinity and glass transition temperature. This can be achieved by increasing the segmental motion of the chain through the addition of a third component. The addition of plasticizers, additives, salts, or inorganic nanoparticles offers a wide range of properties to play with; chemistry and concentration can drastically tune the final properties.
In this regard, the polymer electrolyte research line has designed a wide gallery of lithium salts, in which the lithium ionic conductivity and the solid electrolyte interface have been significantly improved.
Composite/hybrid electrolytes are named when inorganic nanoparticles, polymer and salt are mixed. In the European SAFELiMOVE project, coordinated by CIC energiGUNE, hybrid electrolytes are being designed. The project aims to develop a new solid-state battery technology for electric vehicles that is safer, has a longer range, shorter charging time and is cheaper.
As it can be deduced, a single polymer cannot fulfil all requirements. Polymer synthesis is a useful tool to tailor the properties of polymer electrolytes. At CIC energiGUNE we work on different synthesis and strategies to develop a solid-state electrolyte which meet the battery requirements set by the automotive industry for electric vehicles.