Over the last decade researchers worldwide have focused their efforts on developing “beyond lithium” battery technologies to augment, or in certain situations replace, Li-ion batteries. Energy demand is increasing and new solutions are necessary to allow the use of renewable energies, thus enabling a clean transition towards decarbonisation. In this scenario, metal-oxygen/air (M-O2/air) batteries have the capability to play an important role in the development of both stationary and mobile applications, due to their high theoretical energy density compared to current systems.
The inherent advantage of metal-air batteries is that the active material in the cathode is absorbed from the surrounding environment (O2), giving metal-air batteries some of the highest theoretical energy densities of all battery systems.
Among the different chemistries, the Na-air/O2 battery (NAB) is a promising candidate with an energy density of up to 6 times higher than Lithium-ion batteries (LIBs) (~1600 Wh kg-1 vs 250 Wh kg-1). In addition, sodium is the 6th most abundant element in the earth´s crust, which is a significant economic and resource advantage, especially when combined with the option of employing an aluminium current collector, which lowers overall production costs and battery weight.
Peled et al. reported the first NAB in 2011 and since then the research efforts have focused on understanding the mechanisms behind this chemistry which rely on the oxygen reduction reaction (ORR) during discharge and oxygen evolution reaction (OER) during charge, apart from investigating novel electrodes and electrolytes.
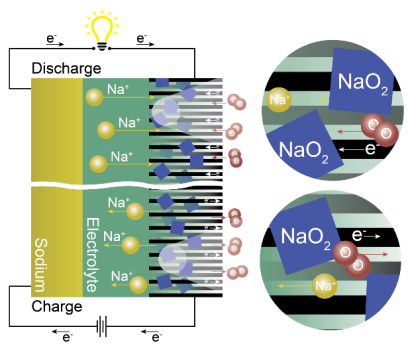
Unlike Li-ion batteries which rely on intercalation chemistry (a cation is intercalated in the electrodes), a NAB store its energy by the so-called conversion reaction. This means that a new solid is formed, i.e., an oxide. Typically, the configuration of the battery consists of a sodium-containing anode, a sodium-conducting organic electrolyte, and an air cathode (see Figure). The reaction mechanism involves oxygen gas dissolving into the electrolyte and forming O22-, which then combines with Na+ to form sodium superoxide (NaO2) (Na+ + O2 + 1e- ↔ NaO2). Other discharge products, such as sodium peroxide (Na2O2) and peroxide hydrate (Na2O2·H2O), have also been described.