Hydrogen is set to play an evermore important part of plans to decarbonise future global energy production and distribution. One of its important roles is as an energy vector, i.e., as a carrier of energy from a primary energy source to where that energy is needed. One major advantage is that when hydrogen is used as a fuel, for example in fuel cells, it is very clean. Water is the only product, and it is free from the emission of harmful pollutants produced in the combustion of hydrocarbon fuels, making it ideal for use in urban environments for heavy transport such as busses and for combined heat and power units in domestic and commercial buildings.
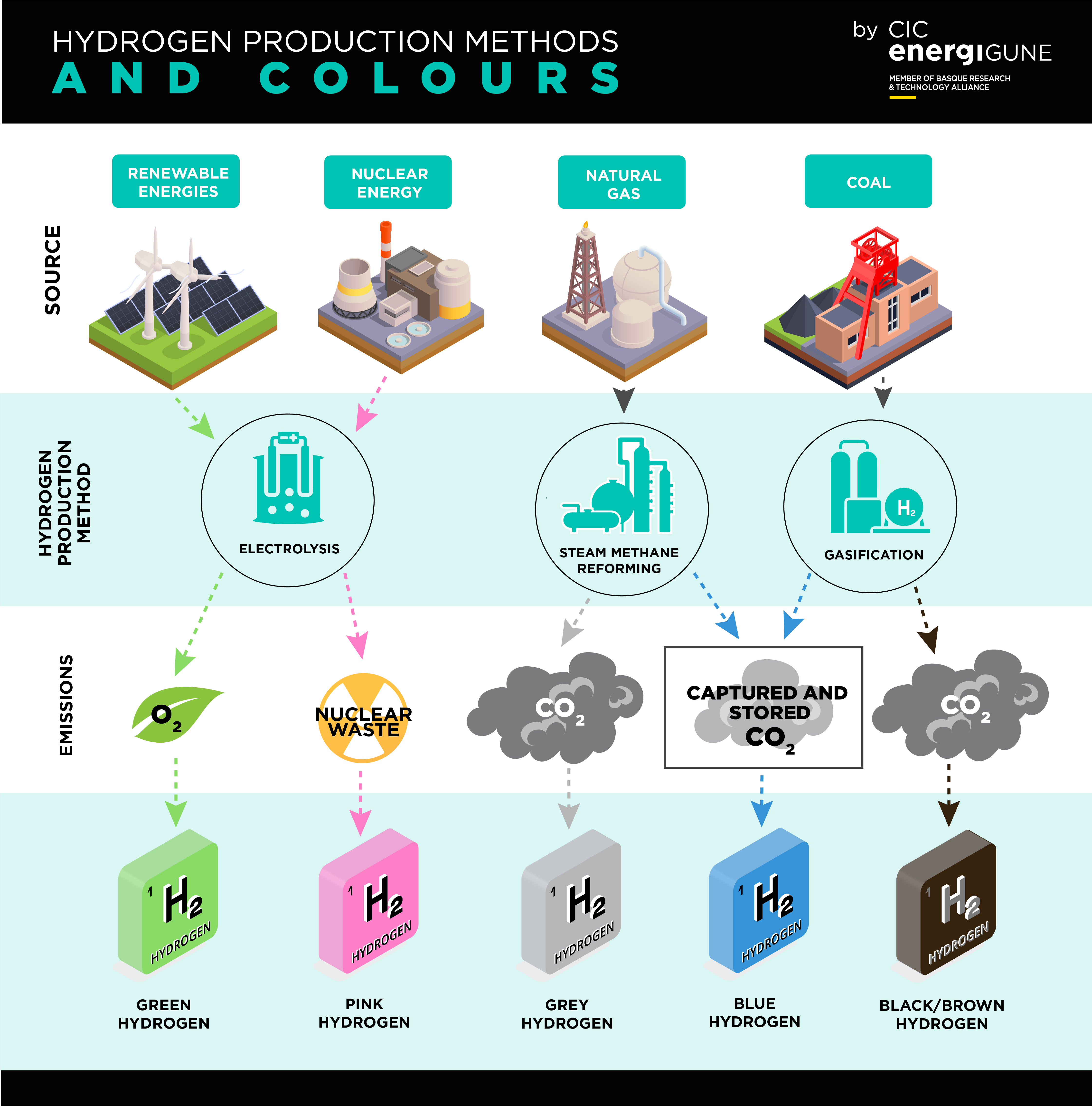
Hydrogen has another important role as a chemical feedstock, in fact the overwhelming current use of hydrogen is for its use in refineries, the production of ammonia for fertiliser and in the production of steel. The International Energy Agency (IEA) estimated that in 2018 the global hydrogen production amounted to 74 million tonnes. Currently hydrogen production is dominated (95%) by processes using natural gas (methane), resulting in the emission of 830 million tonnes of carbon dioxide (CO2) as a by-product. There is thus an urgent need is to find ways to decarbonise hydrogen production to meet global CO2 emission targets.
The production of hydrogen
Although hydrogen is abundant in the universe, free hydrogen is not readily found on Earth. Here it is found bound into molecules, the most abundant of which are water (H2O) and hydrocarbons, especially methane (CH4). To obtain free hydrogen these molecules must be split by providing energy, which can then later be recouped by a recombination with oxygen to produce water. Hence the description of hydrogen as an energy vector rather than a primary source of energy.
How can hydrogen be produced? There are many routes that involve different sources of primary energy which can be in the form of light, heat, electrical energy or combinations of these sources. This blog article is too limited to cover all the various methods of hydrogen production, either in use or being developed, instead it will cover the main contenders for large scale application.
The first, and by far the most universal method is by the steam reforming of natural gas, often known as Steam Methane Reformation (SMR). Here, steam and natural gas, treated to remove contaminants, are heated together at high pressure at ~ 900°C over a nickel-based catalyst. The result is a mixture of carbon monoxide (CO) and hydrogen known as syngas. Syngas is further treated in the water-gas shift reaction to produce more hydrogen and carbon dioxide (see box for details). The CO2 produced is currently released into the atmosphere but can be used in as a by-product e.g. in food processing and packaging where, again, it is ultimately released into the atmosphere. The steam reforming process derives the heat necessary to drive the reaction from the combustion of natural gas producing more CO2.
|
Steam Methane Reformation At high temperatures and pressures methane and steam will react as described in the equation below; CH4 + H2O -> CO + 3H2 The product of hydrogen (H2) and carbon monoxide (CO) is called syngas and can be used to synthesise other products. More hydrogen can be produced by subjecting the syngas to the water-gas shift reaction, shown below, at lower temperatures. CO + H2O -> CO2 + H2 The overall reaction of the process is then CH4 + 2H2O -> 2CO2 + 4H2 With the methane and the water each supplying 2 molecules of hydrogen. The hydrogen is extracted from this gas mix by a process known as pressure swing adsorption which adds to the energy needed (and hence CO2 emission). |
Another route to providing the heat for the SMR reaction is by a process known as partial oxidation of methane, which evolves heat as the reaction takes place. The partial oxidation reaction can be coupled to the steam reforming reaction to make what is known as an autothermal reformer (ATR) (see box). Because no external heat is required, this is a more efficient and compact reformer meaning lower capital cost and less carbon emissions (see, for example, the Johnson Matthey LCH™ process).
To decarbonise these methane-based processes, the CO2 produced must be captured and then stored in a geological repository. Carbon Capture Usage and Storage (CCUS) projects involve pumping the CO2 into geological reservoirs, such as depleted oil and gas fields. An example is the development of the hydrogen network HyNet in the northwest of the UK.
|
Partial Oxidation and Autothermal reforming Partial oxidation is another route to produce hydrogen. Methane is mixed with sufficient oxygen or air to produce syngas. This can again be further processed by the water-gas shift reaction to produce more hydrogen. 2CH4 + O2 -> 2CO + 4H2 This reaction produces heat, which can be used to drive the SMR reaction. If sufficient quantities of steam methane and oxygen are mixed at high pressure and high temperature autothermal reforming can be promoted. 3CH4 + O2 + H2O -> 3CO + 7H2 |
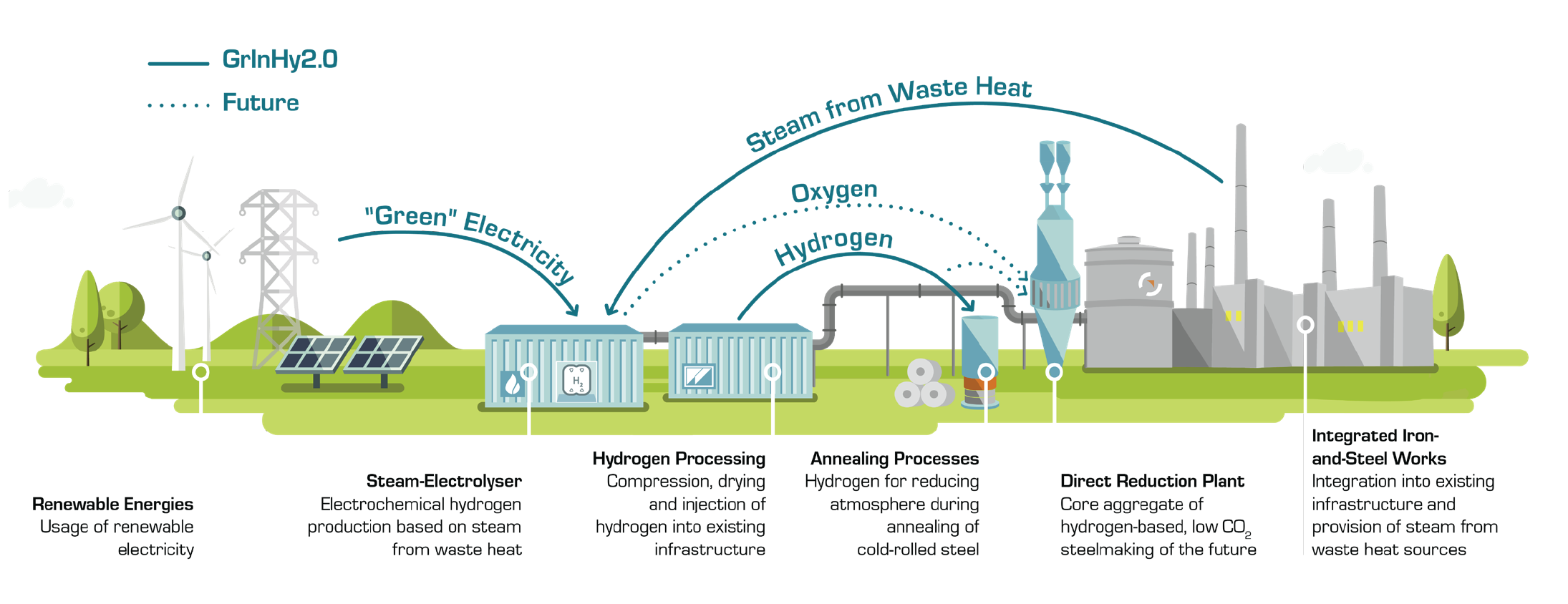
The alternative route that is being strongly promoted is the production of hydrogen by the electrolysis of water, especially if renewable electricity can be used. This was described in a previous blog as the production of green hydrogen. Projects such as GrInHy2.0 are targeted at the production of green hydrogen for use as an industrial feedstock.