Interfaces
Let’s face it, we all have to interact. Our entire universe revolves around interactions. From the sensation of touch to the catalysis of chemical reactions to the waterproof coat you might wear on a rainy day, all rely on a specific behaviour at an interface.
These interfaces are of enormous interest to researchers in pretty much all fields, notably catalysis, material separation, and energy storage and conversion. Moreover, it is at the nano-scale that things get truly exciting: the surface area of nanomaterials is immense in comparison to macroscale counterparts, maximising interfacial interactions, and, upon intrusion/extrusion of liquids, they can exhibit unique properties that possess the potential to facilitate the otherwise impossible.
The energy of interfacial phenomena can be optimised and harnessed for chromatography, smart nanomachines, and energy storage applications. There are myriad potential applications to obtain, recycle, and redistribute energy and control systems with smart materials. Due to the current limitations of electric vehicles, for example, the possibility of saving energy from waste heat is particularly appealing, as is the conversion of other forms of energy.
Here at CIC energiGUNE, the research group Interfacial Phenomena and Porous Media study these phenomena to adapt and optimise them for a plethora of applications, such as the flagship project triboelectrification of porous materials as part of the Electro-Intrusion project (FET-PROACTIVE of the Horizon 2020 Programme) and the development of aqueous HPLC technology with the NoDRY ERC proof of concept project, in tandem with computational investigations conducted at Sapienza Università di Roma.
Intrusion/Extrusion
Let’s get into it! Porous nanomaterials are being investigated for a wide range of applications, such as shock-absorbers and bumpers, molecular springs, thermally-regulated artificial muscles (actuators), smart valves, chromatography, smart fluids, and even porous water!
They possess large volumes and enormous surface areas for their size, making them promising storage media for gases, medicine, and even lithium-ions in potential battery technologies. Some can compress and expand under specific conditions, allowing them to behave like smart valves, switches, and artificial muscles. On the other hand, there are others that are hydrophobic and possess fascinating properties when forced to interact with water that can be exploited for a wide variety of energy applications, such as shock-absorbers, molecular springs, and triboelectric generators.
Intrusion is the entry of a material into a cavity or channel under pressure, and extrusion is the reverse process. One of the main focuses of research is storage and separation. Porous materials are also commonly used in column chromatography, where materials are separated for purification in the lab or in industrial settings, and there is even work being done on drug delivery, where porous materials can retain a specific drug/chemical until a specific trigger in vivo (e.g., temperature, pH) causes it to be released into the body. In addition, some porous materials can even be synthesised around drug molecules, avoiding the step of intruding the material.
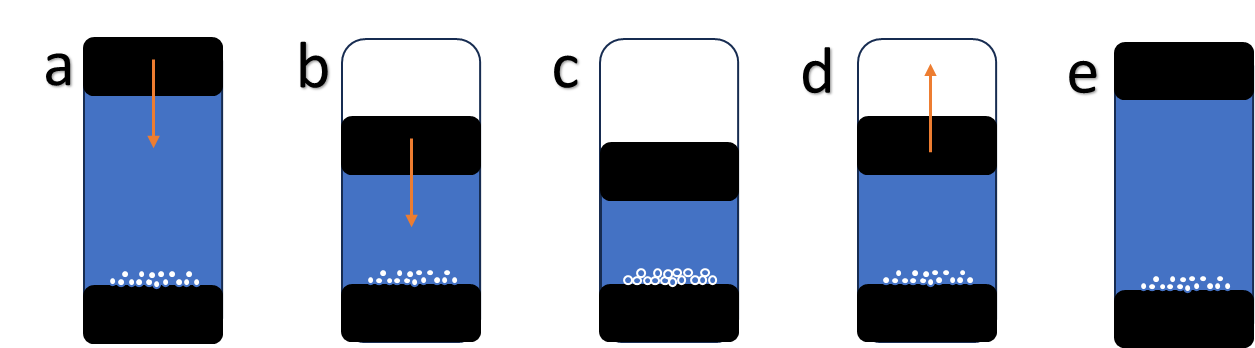
In Figure 1, the intrusion/extrusion of water into the material is illustrated schematically, with water entering the porous material at sufficiently high pressure (1c). Depending on the flexibility of the material (amongst other factors) there can be different degrees of energy loss during cycling of intrusion/extrusion. Whilst this may be energy lost if the system is considered as a battery, it is advantageous for a shock absorber, in which energy needs to be dissipated effectively.