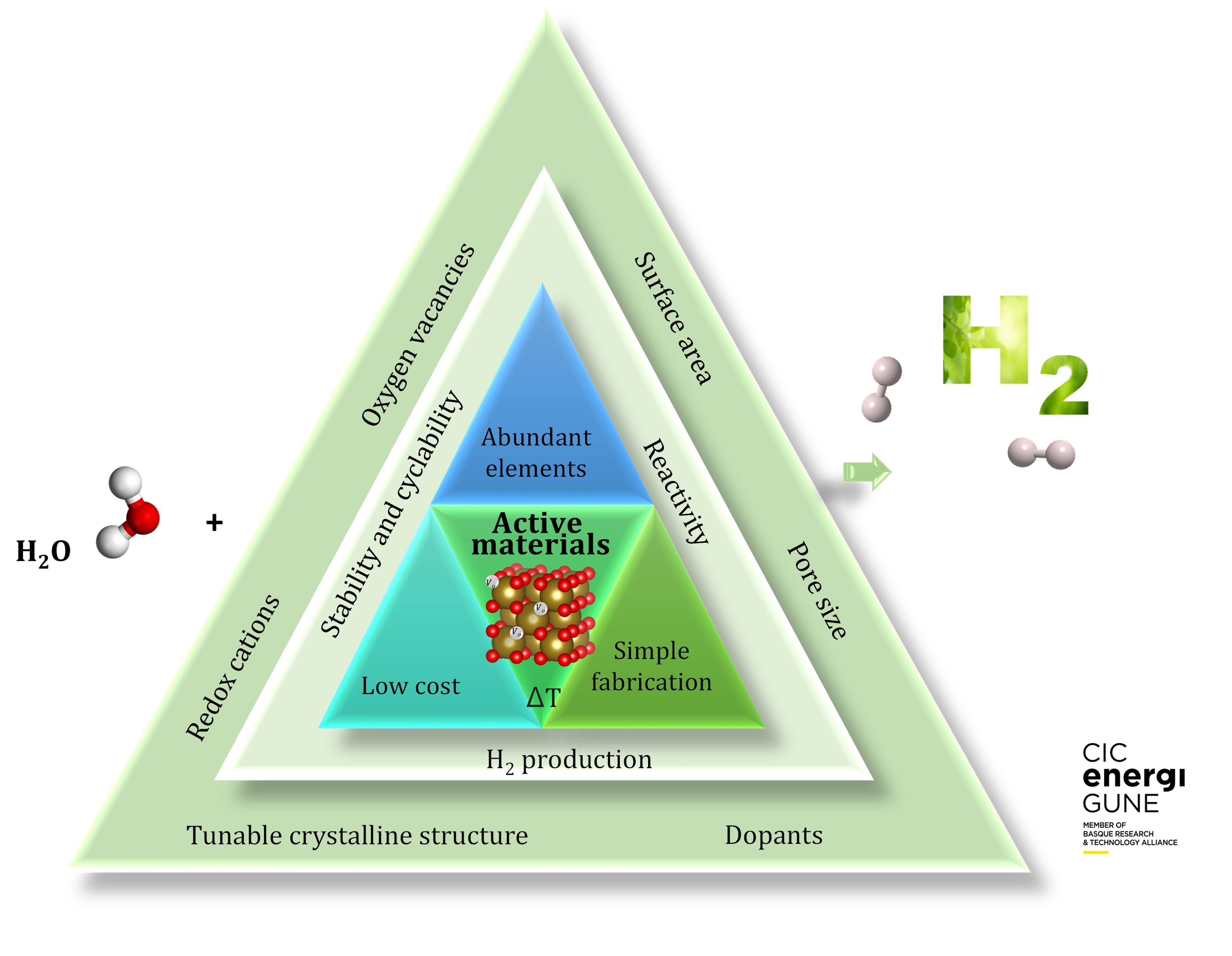
Abundance and cost
For achieving sustainable and cost-effective scalable process, robust earth-abundant based materials showing high performance and cyclability for H2 production are required.
Oxygen exchange capacity
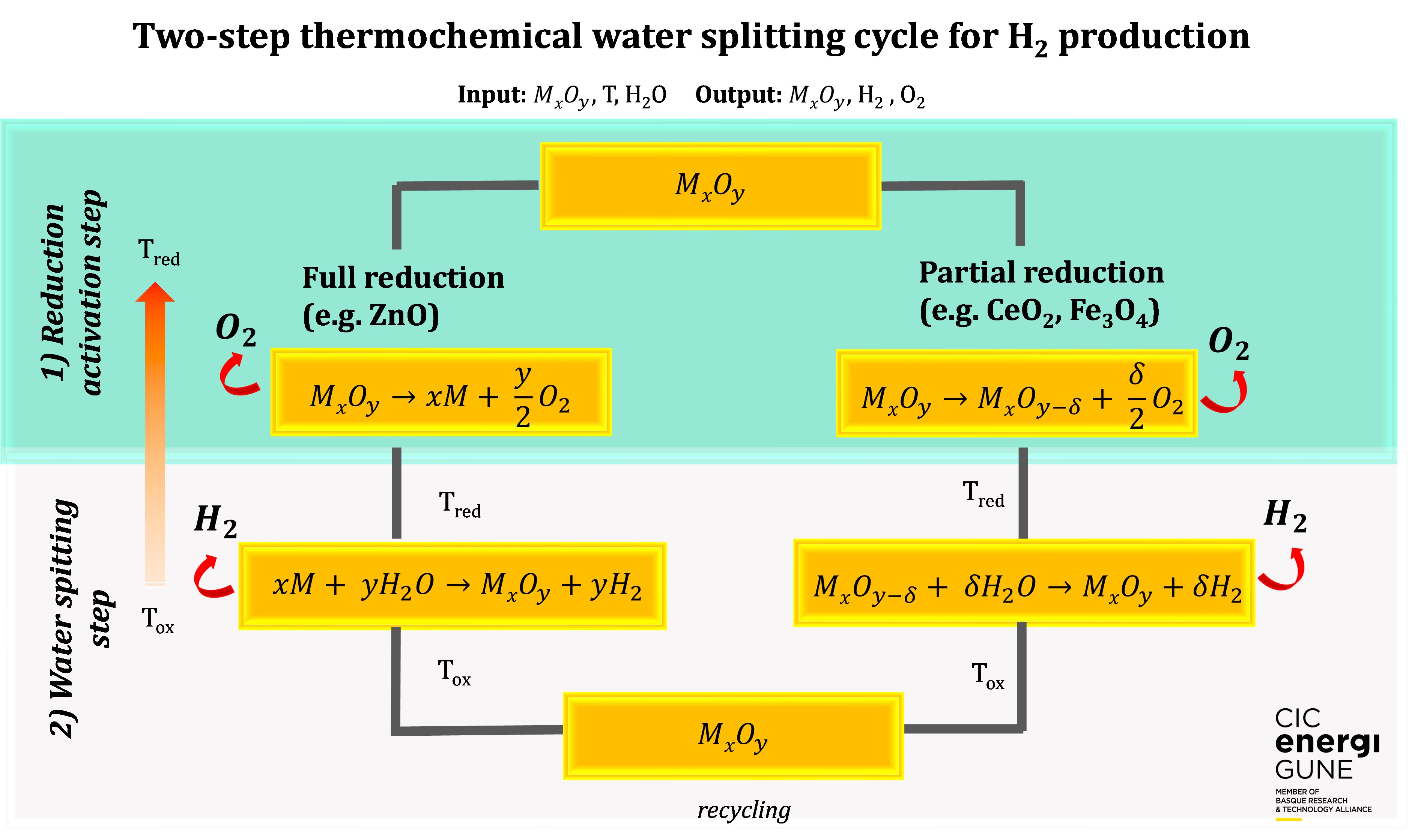
The amount of oxygen released in the reduction step depends on the oxygen exchange capacity of the material and it influences the maximum amount of fuel produced in the oxidation step. The oxygen exchange capacity is defined by the non-stoichiometry (d).
The goal is to have materials with high oxygen exchange capacities and high stability over a large number of cycles6.
Volatile – non-volatile
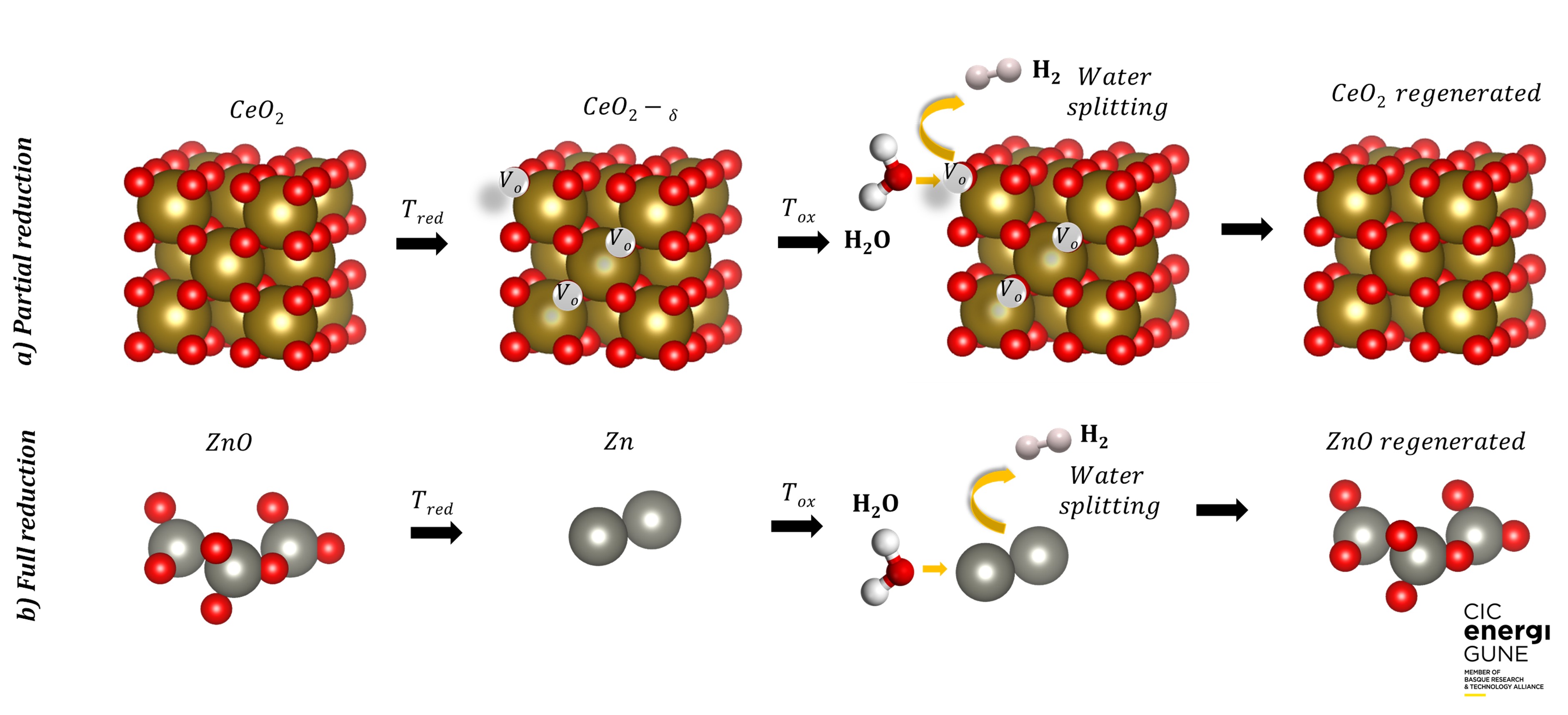
The cycles based on metal oxides can be categorized in volatile (e.g. ZnO/Zn) or non-volatile (e.g. Fe3O4/FeO), depending if the oxide undergoes solid-gas transition, or remains in solid phase during the reactions. The oxygen released and the fuel produced in volatile systems is higher compared to the non-volatile. However, after the reduction step the material should be quenched to prevent recombination7.
Stoichiometric – non-stoichiometric
Meanwhile, the non-volatile systems can be classified in stoichiometric (e.g. Fe3O4) and non-stoichiometric oxides (CeO2), and the thermodynamic and kinetic properties of these oxides are affected by doping or substitution by anions and cations. In a general way, the stoichiometric oxides exhibit higher oxygen release, but their disadvantage is a slow kinetic and low structural and chemical stability, which hinders the performance of the material and limits the amount of fuel produced7.
Crystalline structure
Materials that offer stable crystalline structure with tuneable composition are highly attractive since they offer the possibility of doping and oxygen vacancy formation with no phase transformation after the redox reactions. The most studied families include perovskites, spinels, and pyrochlores, among others8.
Redox activity
The redox capacity is highly influenced by the electronic structure of the material, and cations in the crystalline structure play a determinant role during the redox reactions. For instance, materials with cerium, iron, manganese, lanthanum, and other transition metals have shown high reducibility. However, an ideal material must exhibit appropriate thermodynamic and kinetic properties for both reduction and oxidation steps, which is still a challenge since not all the materials show fast oxidation kinetics5.
Doping
The effect of doping on the properties of metal oxides for thermochemical water splitting has been studied in deep. For instance, it has been proved that substituting cerium by divalent, trivalent and tetravalent dopants of smaller radius is beneficial to promote higher amount of oxygen released. Meanwhile, a dopant with higher valence and smaller ionic radius favors the reduction extent of doped ceria, modifying the M-O bonds in the crystalline structure, which are more easily breakable in these conditions9.
Surface area and pore size
Materials exhibiting microporous structure benefit the oxidation reaction, due to the high surface area, while materials with macroporous structure, with pores in the range of millimetres, favour homogeneous heating5. Also, it has been shown that the use of nanoparticles enhances the H2 production due to higher surface exposed area, improving the reaction kinetics, the heat and mass transfer and the overall reaction rate. Table 1 summarizes the advantages of porous materials for thermochemical water splitting.
Table 1. Advantages of porous materials for thermochemical water splitting.
| Characteristic |
Non porous |
Porous materials |
| Sintering |
+++ |
++ |
| Kinetics |
+ |
+++ |
| Heating homogeneity |
+ |
+++ |
| Mass transfer |
++ |
+++ |
|
Reactivity solid-gas
|
+ |
+++ |
| Pressure drops |
+++ |
+ |
| Reactant and products difussion |
+ |
+++ |
Perspectives
Nowadays, the main limitations regarding the active materials for thermochemical water splitting are associated with the high reduction temperature, and the low H2 production and cyclability of the materials, due to undesired processes including sintering, low reactivity, and secondary phases formation. For this reason, in CIC energiGUNE, we are working in the design and development of new and competitive materials that can offer stable H2 production and large lifetime as prospects for their use in large-scale H2 production.