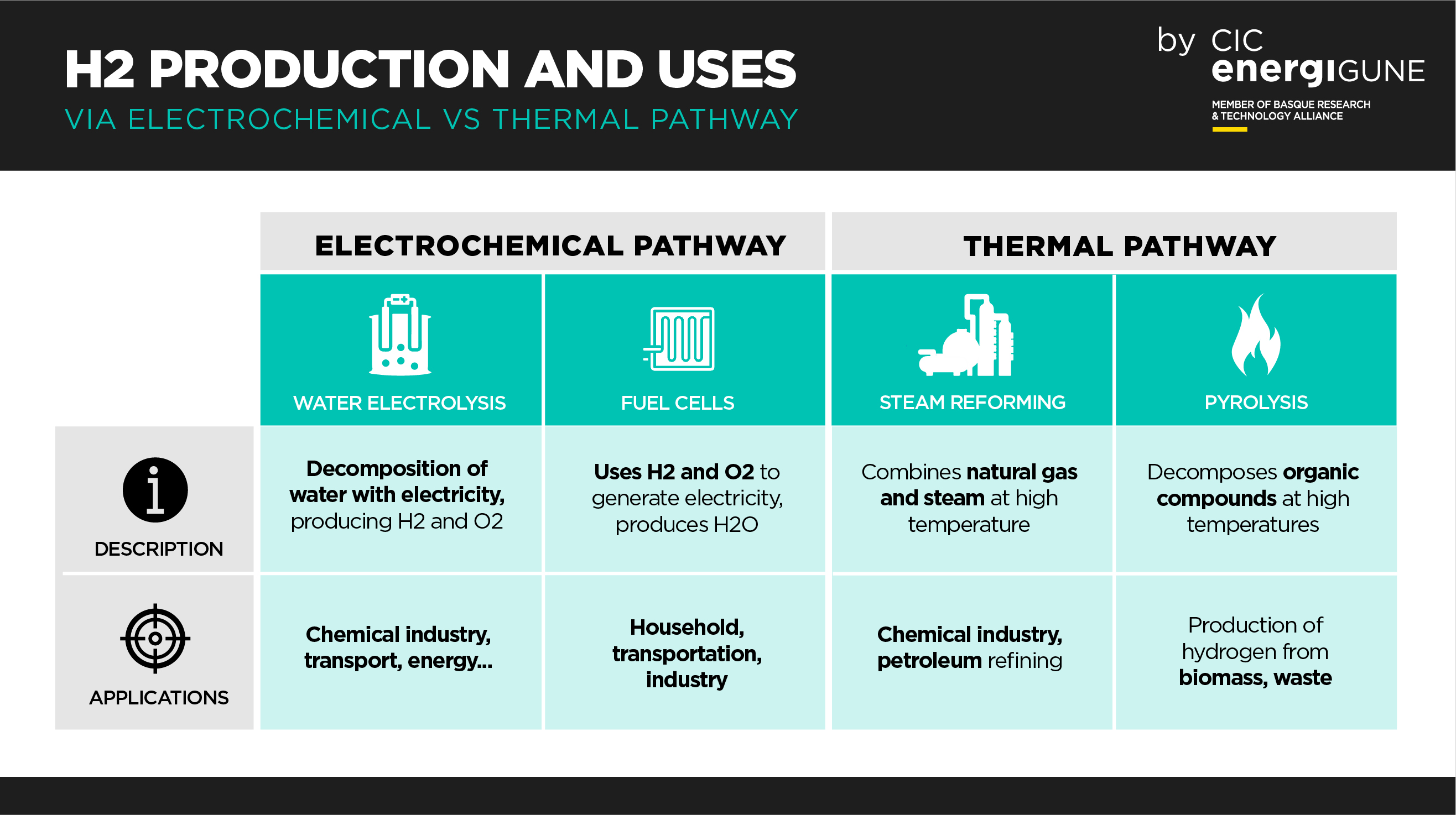
Electrochemical Hydrogen Production:
The electrochemical pathway is a process in which electricity is used to break down a water source or a hydrogen-containing compound into its basic components, hydrogen (H2) and oxygen (O2). Two of the most common methods are water electrolysis and fuel cells.
Water Electrolysis:
One of the best known and most widely used processes in the production of hydrogen by electrochemical means is the electrolysis of water. This process involves passing an electric current through a solution of water or water vapor, resulting in the separation of water into hydrogen at the cathode and oxygen at the anode.
This process is efficient and produces pure hydrogen, making it suitable for applications where high-purity hydrogen is required, such as the chemical or semiconductor industries. In addition, water electrolysis can be powered by electricity generated from renewable sources, making it a sustainable way to produce hydrogen, known as "green hydrogen".
Fuel Cells:
One important method of producing electricity from hydrogen electrochemically involves fuel cells. In this case, fuel cells use hydrogen and oxygen from the air to produce electricity, water and heat as by-products. Fuel cells are used in power generation applications and hydrogen vehicles.
This process is highly efficient and produces no carbon emissions when using hydrogen produced from clean sources. Fuel cells are particularly suitable for mobility applications, as they offer a fast and efficient way to convert hydrogen into electricity to power fuel cell electric vehicles.
Thermal Hydrogen Production:
In contrast, thermal hydrogen production involves the use of heat instead of electricity. A common method is steam reforming, in which a hydrocarbon, such as natural gas, is combined with water vapor at high temperature in the presence of a catalyst to produce hydrogen and carbon monoxide (CO).
The carbon monoxide can then be removed to obtain pure hydrogen. This method is widely used in industry for large-scale hydrogen production.
Another thermal method is pyrolysis, which decomposes organic compounds at high temperatures in the absence of oxygen to obtain hydrogen and other gaseous products. Although less common than steam reforming, pyrolysis has applications in the production of hydrogen from organic waste and biomass.