There is still no unanimity within the industry as to which electrolyte may be the "winner", since all these alternatives have different advantages and areas of improvement that in many cases raise the possibility that in the future they may coexist in order to be used in different applications according to the need.
Hence, the different companies working in the development of solid-state technologies have their own bet and value proposition, depending on the end market they aim to satisfy.
Let´s remember that solid state technologies are not only intended to be the critical disruption in industries such as electric vehicles (where most attention is currently being focused); but also in other applications such as consumer electronics, stationary applications aimed at boosting renewable energies or even other means of transportation such as aviation (as we have already seen in our social networks) or the railway sector.
What properties does the use of one or the other alternative present, as well as advantages depending on the application? These are detailed below:
HIGH DENSITY AT COMPETITIVE COST
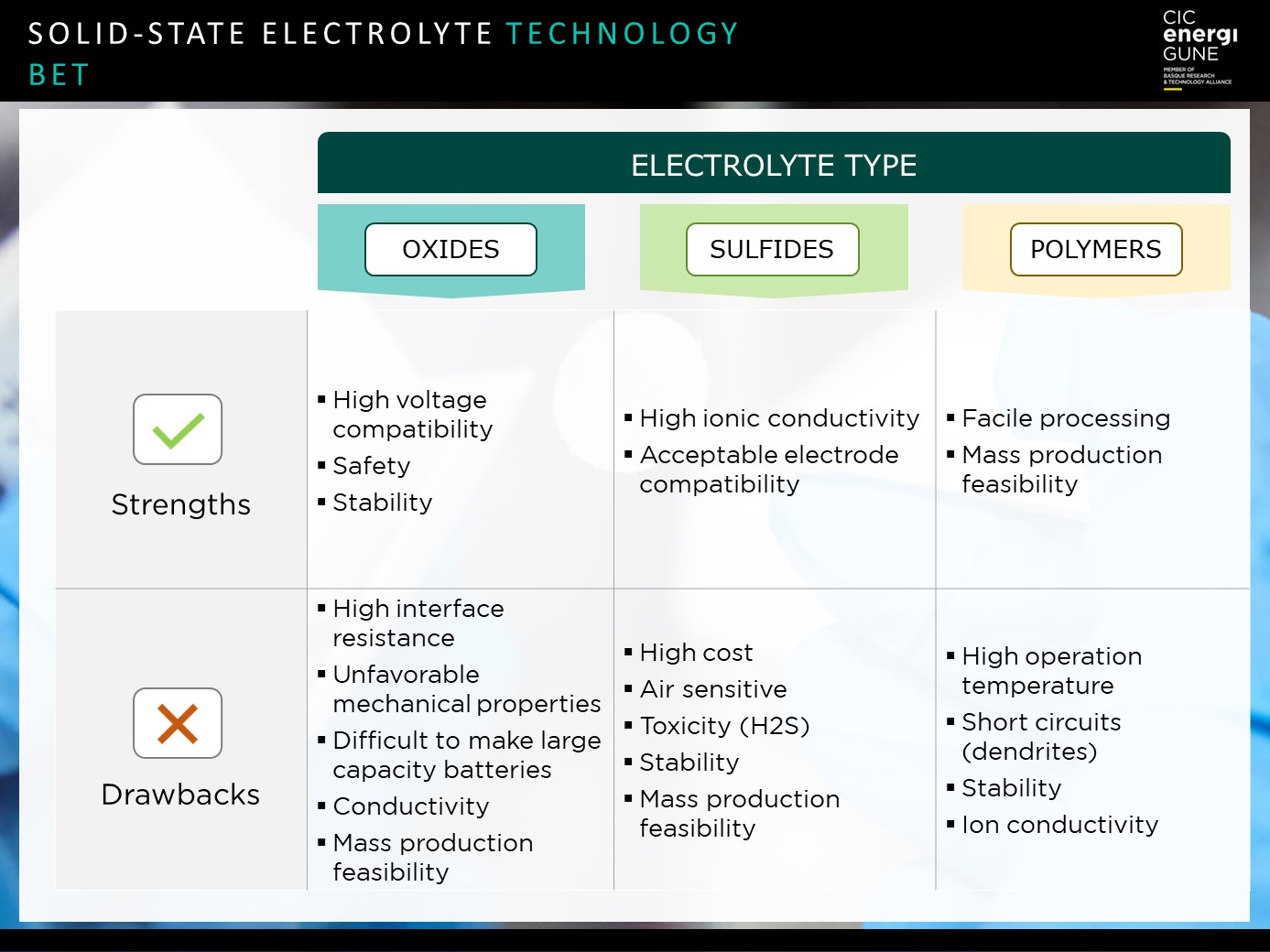
Polymer-based electrolytes currently appear to be the big bet for the mass electrification of industries such as electric vehicles. This is due to two of their main strengths over other alternatives.
On the one hand, they have a high compatibility with Li metal as an anode of high specific capacity, which translates into greater autonomy and range of kilometers traveled (a key indicator in electric vehicles).
On the other hand, there is their potential for industrialization in larger cell formats, as well as their scalability thanks to the greater ease of processing inherent to plastic materials. All this results in a more competitive cost, which is once again a key property for achieving affordable electric vehicles that are accessible to different market segments.
In addition, the use of polymeric materials reduces the flammability of the battery, thus achieving greater safety.
However, there are some technical challenges to be faced for their mass industrialization. Above all, in relation to the ionic conductivity of this type of electrolytes, diminished by the semi-crystalline nature of the polymers. This requires increasing the operating temperature to values above 60º, which implies the need for battery management systems capable of self-regulating this temperature for optimal use.
OXIDES: STABILITY AND SAFETY
In the case of solid electrolytes based on oxides, we are faced with an alternative that is at the opposite end of the spectrum to polymer solutions in terms of properties and advantages.
Above all, the strengths of this technology are based on their high mechanical and chemical stability, which makes them compatible both with high specific capacity anodes such as lithium metal, and with high voltage cathodes due to their large electrochemical window. They also have good ionic conductivity (even at low temperatures), all of which results in greater safety and "ease" of management, which is an advantage in terms of use and application.
However, the big challenges are precisely what makes polymers so attractive for large-scale use: the processing and scaling up of their manufacturing process and cost.
Their manufacturing process is more expensive and has fewer synergies with conventional processes, which has an impact on the final cost of production and, therefore, on their price in terms of market access.
SULFIDES: IONIC CONDUCTIVITY IN A POTENTIALLY INDUSTRIALIZABLE SOLUTION
Finally, we find solid electrolytes based on sulfides, a solution that is located at an intermediate point between the alternatives already described, although with a large area of improvement to be solved.
Its outstanding properties are its high ionic conductivity and its mechanical properties in terms of softness and plasticity, which offer advantages for processing - compared to oxides - and, at the same time, facilitate the formation of good contacts - interfaces - with both anodes and cathodes. All this makes it an adaptable solution for a wide range of applications in different conditions of use. In addition, its manufacturing process does not require high-temperature sintering steps, which results in a more affordable final price compared to oxides.
Its durability has also been tested, achieving high-capacity retention rates after 1,000 and 2,000 cycles.
However, the current focus of this alternative is on overcoming the technical challenge associated with its safety. This challenge arises from its high reactivity to air and humidity, which results in the formation of H2S upon decomposition, generating a poisonous gas in high concentrations that puts at risk anyone using or handling this type of technology.
BORATES AND HALIDES: NEW AND PROMISING CANDIDATES
Finally, there are other families of solid electrolytes that are also emerging as possible candidates and raising the interest of the international scientific community: borates and halides.
Borates were initially developed as rocket fuels and hydrogen storage applications. They show good ionic conductivity, acceptable oxidation and reduction stability, and adequate mechanical properties. Their instability in air and, above all, their complicated, inefficient and expensive synthesis process greatly limit their exploitation potential, and their use in laboratory cells has only been demonstrated on very limited occasions.
Halides, on the other hand, are soft materials with processability properties similar to sulfides, with high ionic conductivity and electrochemical stability against oxidation. Their main limitations are their high sensitivity to moisture and their incompatibility with lithium metal as an anode. Halide research can be considered to be in its infancy, although in recent years numerous research groups have reported different cell concepts based on halides with very promising results. With TRLs considerably lower than in the previous cases, their path to commercialization and industrialization is also significantly longer than in the case of polymers, oxides and sulfides.
WHERE WILL THE INDUSTRY GO IN THE FUTURE?
As mentioned at the beginning of the text, the advantages and challenges facing each of these alternatives means that in the future a scenario will arise in which all these solutions will coexist depending on their end use and the needs of those who use them: