In the current economic and environmental global landscape, where the demand for energy storage systems is growing rapidly, batteries are expected to play a key role in a low-carbon economy.
To date, lithium-ion batteries (Li-ion or LIBs) have dominated the market for portable electronic devices and become the leading candidates for electric vehicles, triggering a substantial growth in global LIB production.
On the one hand this has led to decreasing costs, but on the other hand it has also caused an increase in the mismatch between the supply and demand of raw materials which are preferably avoided – such as those with limited availability or health risks (i.e. are critical and/or toxic).
Two such materials are graphite and cobalt, which are considered to have vulnerable supply chains. Consequently, an increase in LIB production will lead to new environmental and social challenges, which will rapidly become more important as the market expands.
In such a scenario, it will be increasingly vital to search for new, efficient, and cost-effective strategies for the reuse and recycling of LIBs, as well as for new alternative energy storage systems based on abundant, sustainable and low-cost materials (which may then be used an alternative to lithium-ion batteries wherever suitable).
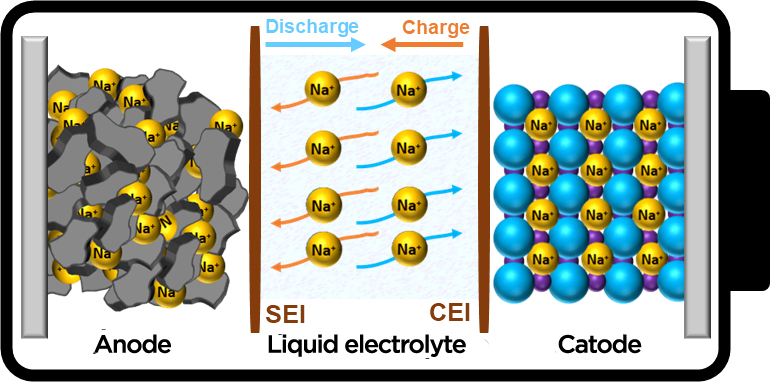
Within this framework, and with the aim of being able to meet both existing and future needs, different energy storage technologies called "post-lithium or beyond-lithium" have appeared in recent years - among which are sodium-ion batteries (Na-ion or SIBs).
Sodium as an alternative, sustainable battery technology
Sodium is a is low-cost and abundant chemical element, homogeneously distributed around the world. Given this relative wealth of necessary resources and components, as well as the limited use of critical materials and the low starting cost of their raw materials, SIBs have clear advantages over many current batteries - such as LIBs, lead-acid (Pb-Acid) or nickel-cadmium (Ni-Cd) batteries, as shown in Table 1 below.