Advantages and challenges of latent heat storage
All of the technologies mentioned above store/release energy by increasing/decreasing the temperature of the storage material (sensible heat storage) and therefore they provide low compact storage solutions (low storage capacity per unit volume). Achieving a significant increase in compactness without increasing the investment cost is one of the current R&D challenges.
Towards this end, the scientific community is working on developing technologies that use phase change processes (latent heat storage) or reversible thermochemical reactions (thermochemical storage) as the basic principle for storing energy. Thermochemical storage has a very high potential to achieve ultracompact systems but is still at an early stage of development, leads to complex storage systems, and the associated costs are unaffordable for the moment. In contrast, latent heat storage has undergone further development and involves much less sophisticated system technology. However, although it can meet the desired compactness objectives, the investment costs are still significantly higher than those of sensible heat storage technologies.
Contrary to what one might think, it is often not the storage material, but the heat exchanger, which is the most expensive element. Indeed, the most commonly used phase change materials are low-cost anhydrous salts, which store thermal energy during melting and return it during the reverse solidification process, and which are characterized by high enthalpies of fusion. However, their thermal conductivity is relatively low and this implies the use of heat exchangers with high exchange surface area to meet the discharge power requirements. In fact, generally, the heat exchanger represents more than 60-70% of the cost of the storage system.
How to combine the need for compactness with low-cost requirements
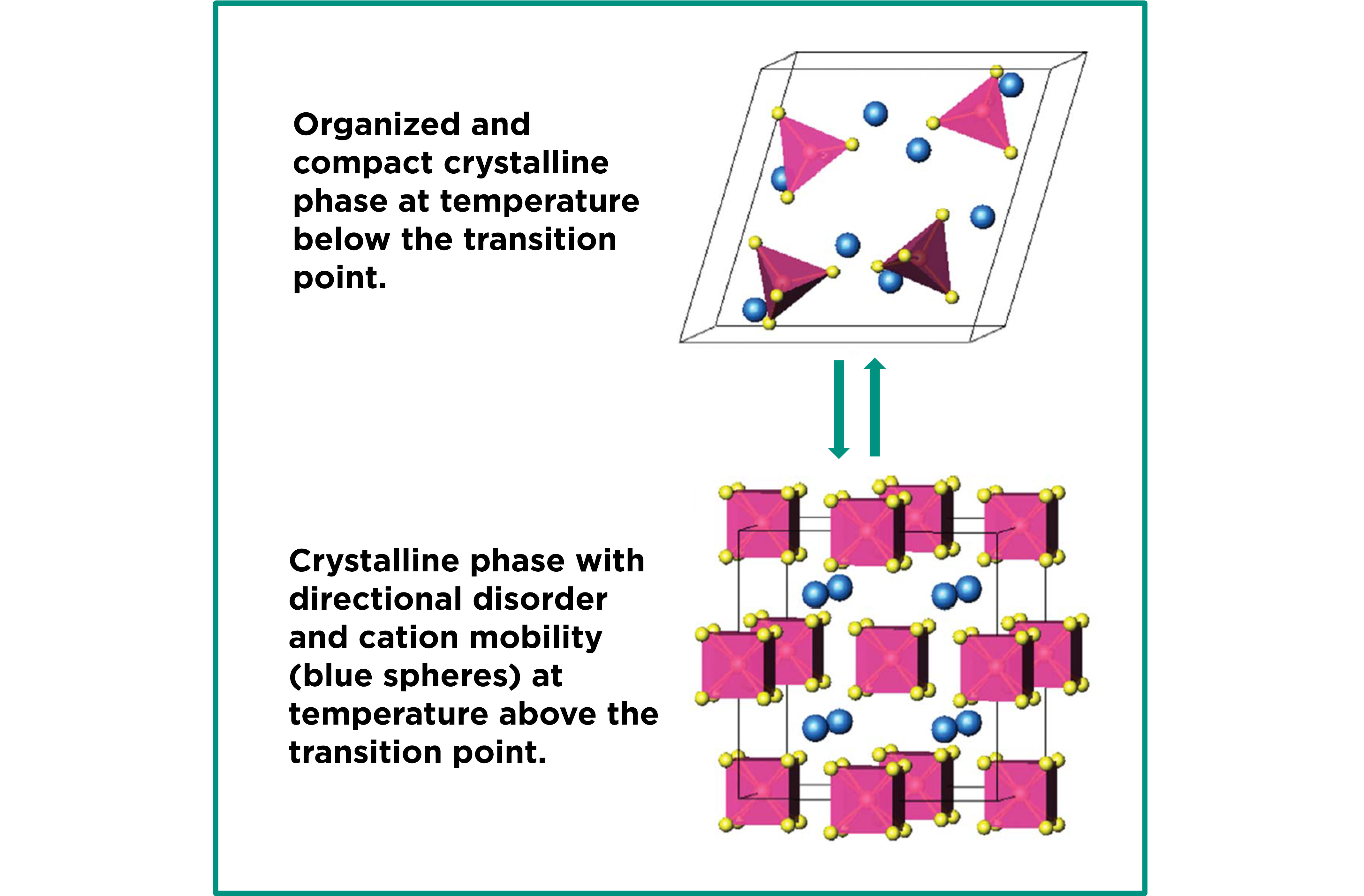
CIC energiGUNE is developing new thermal storage materials that can achieve high levels of compactness at competitive costs. These are inorganic ionic materials that undergo highly energetic solid-state phase transitions. Below the so-called transition temperature, the material organizes itself into a highly ordered and compact crystalline lattice. However, above this temperature, a new crystalline phase appears, which is less compact and characterized by a high degree of directional disorder. The entropy difference between phases is high and, consequently, so is the phase transition enthalpy. This, together with high specific heat values, results in a surprisingly high volumetric thermal storage capacity.
On the other hand, the fact that the material always remains in a solid-state, irrespective of the phase in which it is found, is decisive in terms of cost. It allows the storage system to be considered as a fixed granular bed that does not require a heat exchanger. In addition, this feature of the phase change material enables easy handling and transport, makes its integration into the storage system very versatile as it can be easily produced to the desired size and shape, and minimizes or avoids the typical corrosion problems of salts.
The key to the unprecedented properties of these materials lies in their geometrical and structural features. They are formed by XO4= anions with tetragonal geometry, where the X atom (non-metallic element or metalloid) is placed in the center of the tetrahedron composed of four oxygen atoms to each of which it is bound by covalent bonds, and by cations of the alkali or alkaline earth metal groups. Below the transition temperature, the molecules are organized into crystal lattices (generally monoclinic, trigonal or orthorhombic) where all the atoms have fixed positions. However, above the transition temperature, not only is there a change in the geometry of the unit cell of the crystal lattice, but also the ions are endowed with certain mobility.