Imperfection is a universal feature in crystalline materials
“Crystals are like people: it is the defects in them which tend to make them interesting!” This citation from Prof. Collin Humphreys, physicist specialist of semiconductors (University of Cambridge), also holds true for battery materials. However, the presence of faults in their crystalline structures are often disregarded or oversimplified, for lack of means to properly characterize them.
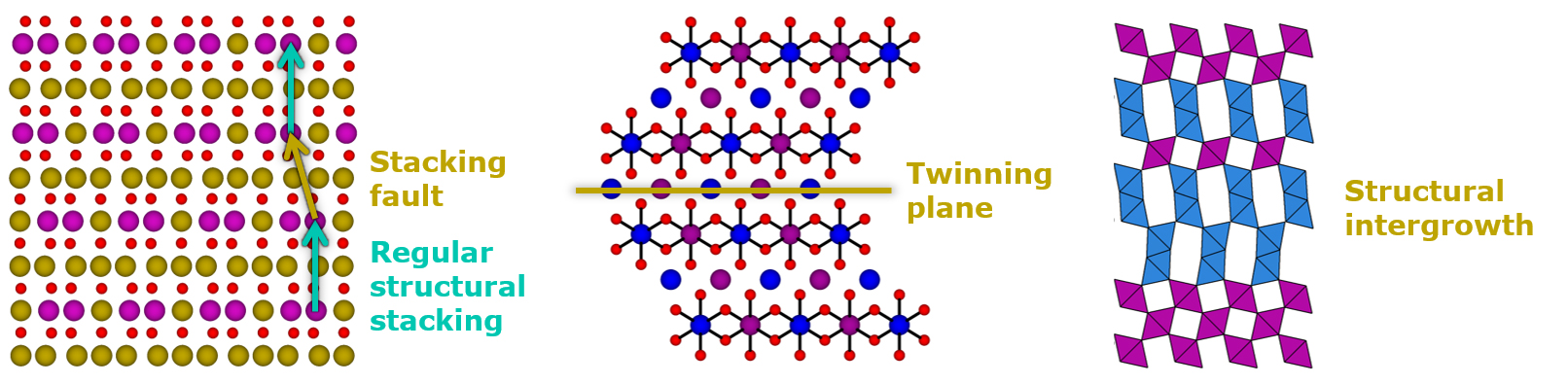
Crystals are generally defined as solids in which the constituent elements are ordered in a specific pattern that is periodically repeated in the three dimensions of the space. Such a definition may suggest that crystalline materials (including most of the electroactive materials for Li-ion and Na-ion batteries) are perfect arrangements of atoms, but in reality, imperfection is a universal feature in crystalline materials.
Crystalline defects generally have bad reputation. By themselves, the names “defect” and “fault” carry a distinctly negative connotation. As a result, they are regarded a priori as detrimental features to functional materials’ properties and performance, and in turn, considerable efforts can be made to either minimize their concentration or to counterbalance their detrimental impact.
However, defects can indeed generate value! The defects and impurities that cause highly desirable color in gem-quality diamonds are in fact a manifest illustration of the opportunity offered by structural imperfections. When renamed “dopants”, defects start to be more attractive. The entire semiconductor industry is indeed built on methods for preparing Si, Ge or GaAs materials doped with precise amounts of desired impurities, which enable to finely tune their electrical properties.
Therefore, in Materials Science, instead of avoiding defects, the intentional and purposely introduction of defects (with control over type, concentration and location) offers novel opportunities for materials engineering. The material scientist can then use them as a tool for tuning and enhancing properties and even enabling new functionalities.
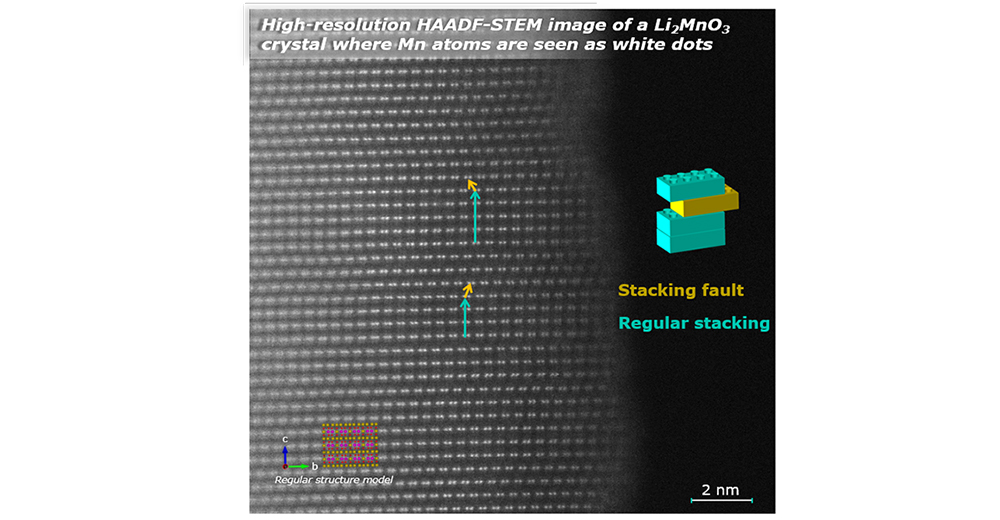
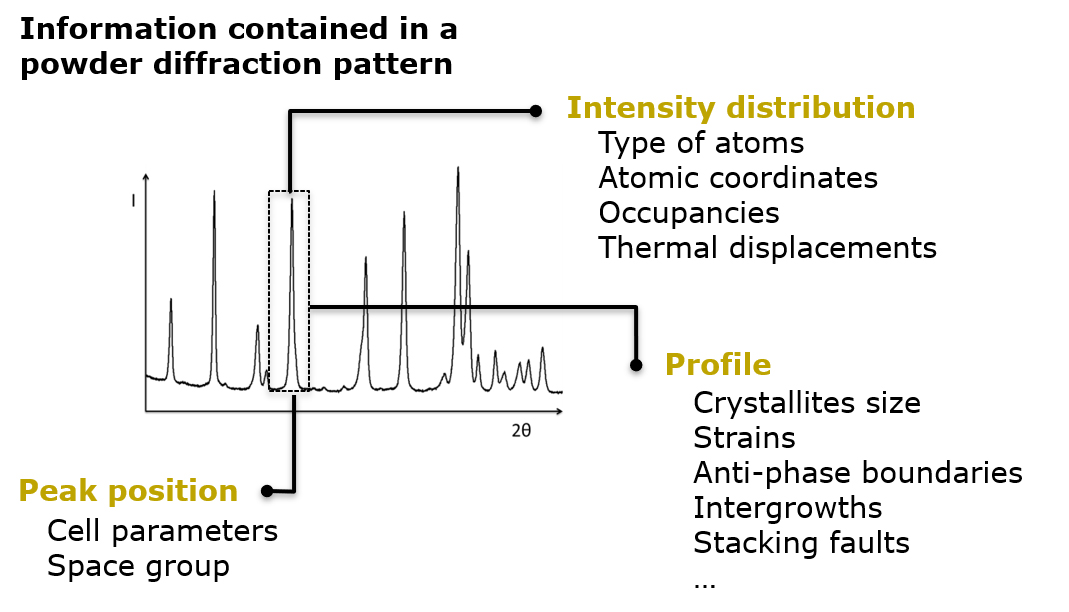
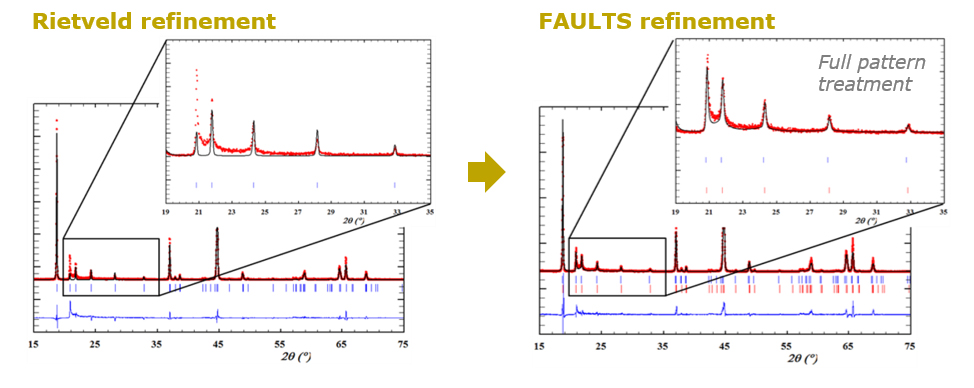
In the field of battery materials, disorder and structural faults are also omnipresent. The challenge resides in accurately characterize the microstructure of the electroactive compounds, understanding the correlations between the microstructural features of the samples and their performance, and eventually control their location and amount during the materials’ synthesis and/or processing, to turn them into advantage and enhance their properties. This is the job of the Crystal Chemist and CIC energiGUNE counts some internationally recognized experts in this field.