Conventional Li-ion batteries present significant risks ought to the flammable nature of the liquid electrolyte. Additionally, Li-ion batteries are close to their practical energy density limit due to the constraints of the carbon-based negative electrode. Therefore, improvements in the electrolyte must follow a complete concept shift approach, rather than only engineering the liquid electrolyte chemistry.
Recent Li-ion electrolyte research has focused on discarding unsafe organic liquid solutions and transitioning towards more inert electrolytes; ideally, solvent-free Li+ conducting electrolytes. Solid polymer electrolytes are inherently safe, significantly decreasing the risk of extensive fires compared to current Li-ion battery liquid electrolytes.
Additionally, solid polymer electrolytes will allow to remove current carbon-based negative electrodes, transitioning toward Li metal and anodeless negative electrodes. This will allow enhancing the energy density of batteries to values which will increase the driving range of electric vehicles up to around 800 km, achieving parity with current internal combustion engines.
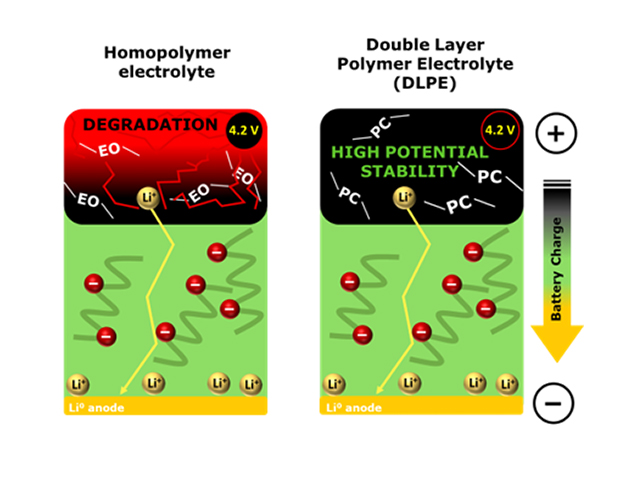
There are different polymer chemistries suitable for battery applications as solid polymer electrolytes. Each polymer chemistry offers different advantages, as discussed in our previous blog post. Nevertheless, it is particularly difficult to develop a polymer electrolyte with a sufficiently large energy gap that offers electrochemical stability against Li metal and high-voltage positive electrode active materials simultaneously.
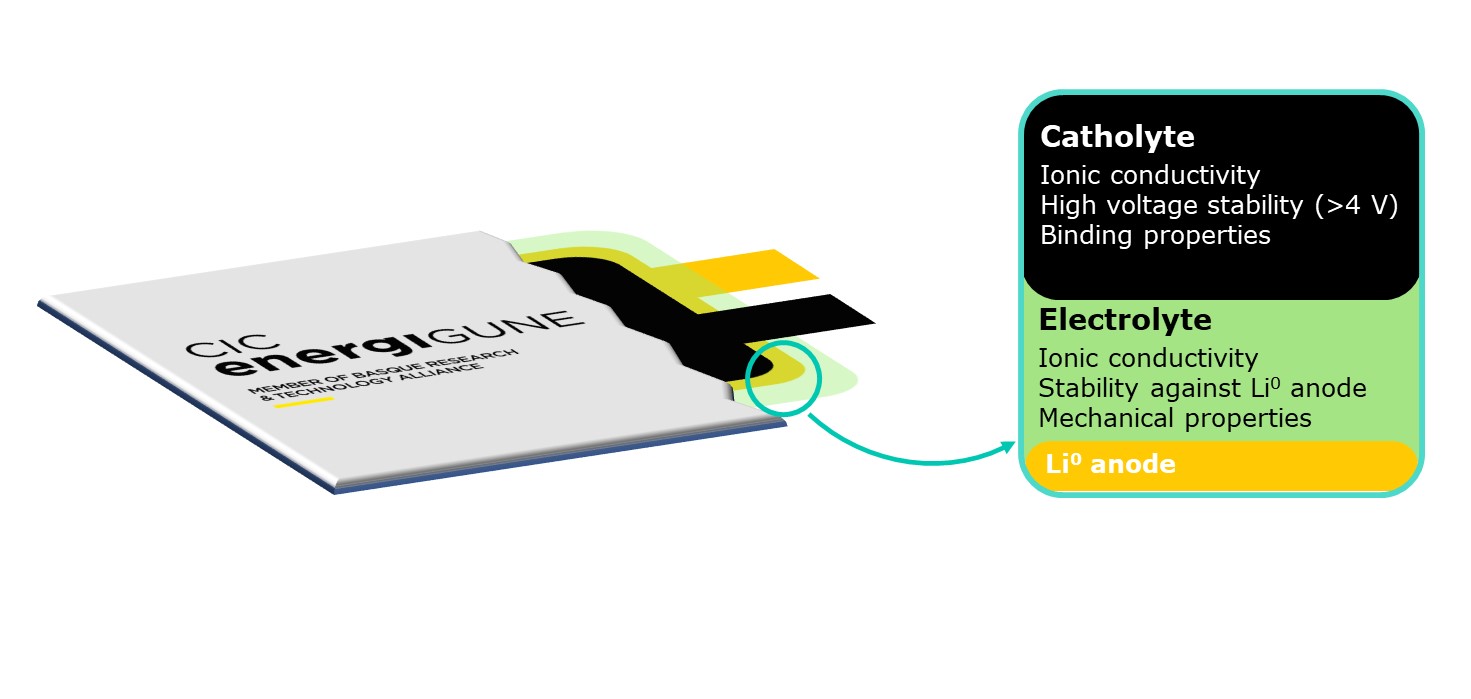
Therefore, CIC energiGUNE has developed a smart and simple strategy combining polymer layers with different properties within the same battery. These layers can be tailored to match the properties required in each battery section. This approach is named Double Layer Polymer Electrolyte (DLPE).
Our research in the last 4 years has focused on developing this Double Layer Polymer Electrolyte technology, where two different polymer electrolytes are used within the same battery: one at the cathode side (catholyte) and one as separator (electrolyte). Each battery section requires different electrochemical and mechanical properties from the polymer; thus, the polymers used in each section may be different. On the one hand, the catholyte requires a high ionic conductivity, stability against oxidative potentials (>4 V vs Li/Li+) and excellent binding properties to maintain all active material particles glued together. On the other hand, the electrolyte requires high ionic conductivity, chemical and electrochemical stability against Li metal negative electrodes, and sufficient mechanical properties to prevent dendrite penetration. Thus, the DLPE approach prevents the undesired degradation of the electrolyte or catholyte that occurs when using a single polymer electrolyte in the whole cell derived from the low energy gap mentioned earlier in the text. Additionally, other properties such as mechanical stability, binding properties and ionic conductivity can be tailored depending on the needs of each battery application.